Reading time: 3 minutes Patty Spears One of the most valuable contributions a patient can make to science is to donate tumor tissue for research. It is important to use the tissue in research in a way that respects the intentions of the patient who donated it. Tumor tissue can continue to add value to... Continue Reading →
Imaging-enabled insights from top radiopharmaceutical therapy trials
Reading time: 5 minutes Bethany Cooper Over the last 5 years, the radiopharmaceutical sector has seen significant global growth, with discoveries transforming cancer diagnostics and treatment through the targeted use of radiation. Methods to deliver radiation are becoming more precise, controlled, and effective, targeting tumor cells directly while sparing healthy tissue. Three seminal trials have... Continue Reading →
Promising trials: Lorlatinib for NSCLC
Reading time: 4 minutes Karli Norville In 2021, lung cancer was ranked as the number one cause of cancer deaths in the United States (1). The American Cancer Society reports that the 5-year survival rate of all Non-Small Cell Lung Cancer (NSCLC) patients is 26% (2). A recent study of the cancer drug Lorlatinib announced... Continue Reading →
A Standing Ovation! Results From DESTINY-Breast04 trial in Breast Cancer
Reading time: 5 minutes Patty Spears The applause was thunderous and traveled like a wave across the large auditorium at the end of a 2022 American Society of Clinical Oncology (ASCO) Annual Meeting Plenary Session talk. It did not stop. The audience stood and kept applauding and reveling in the positive results of a clinical... Continue Reading →
Why Can’t I Be in This Clinical Trial?
Reading time: 5 minutes Patty Spears There are many barriers keeping patients out of clinical trials, even if they want to join the clinical trial. There are multiple steps along the way and each step is a hurdle for patients. A patient must first have physical access to the trial (structural barrier). For example, I... Continue Reading →
Are Clinical Trials in Oncology Biased?
Reading time: 3 minutes Varshit Dusad Randomized controlled clinical trials (RCTs) are an important part of medical studies. Properly supervised trials act as the source of evidence for the safety and efficacy of new drugs, therapies, and medical devices. Hence, it is imperative that clinical trials are designed, conducted, and analysed in a robust and... Continue Reading →
Stakeholders in commercialization of oncology
Reading time: 6 minutes Varshit Dusad Oncology is a vibrant research area with many exciting discoveries unfolding every year, each emerging from research labs all over the world. However, the road from innovations in the lab to successful commercial realization in the real world is long and challenging. For a new therapy and drug to... Continue Reading →
How does a drug get approved?
Reading time: 5 minutes Bekah Schulz The Food and Drug Administration (FDA) is often criticized by patient advocacy groups for taking too much time to approve life-saving drugs. However, the FDA is a difficult situation; if they approve a drug too quickly and it turns out to be unsafe/ineffective, then people are upset. If they... Continue Reading →
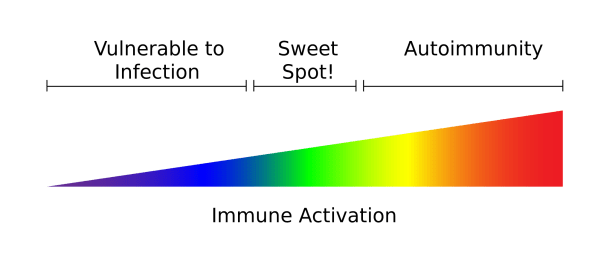
It takes two brakes to stop cancer?
Reading time: 4 minutes Manisit Das In the past, we have discussed checkpoint inhibitors - therapies that block communication between cancer and immune cells, preventing cancer cells from evading the immune system. Dr. James Allison and Dr. Tasuku Honjo, who won the Nobel Prize in Medicine in 2018 for their pioneering work in cancer immunotherapy... Continue Reading →
Participating in a cancer clinical trial: a path rife with difficulties
Reading time: 4 minutes Swetha Srinivasan Cancer therapies, like all other medicines, only make their way to patients after the completion of a lengthy process of extensive research studies involving animals and then people to make sure that the benefits of the drug outweigh the risks. These final research studies in people are commonly known... Continue Reading →
Feeling the ‘heat’ from neighbors: Microenvironment driving cancers in the gut
Manisit Das Not long ago Tamara mentioned in her OncoBites article that it is often hard to determine what factors drive cancer. Even after a mutation responsible for fueling cancer growth is identified, we do not always know how that mutation contributes to tumor formation. Understanding these mechanisms is however quite important. As we gain... Continue Reading →
Stumbling before the beast: Not all cancer clinical trials end in drug approval
Manisit Das Since the beginning of OncoBites, we’ve talked a lot about immunotherapy: using our own immune cells to destroy the cancer cells? We can’t get enough of it! In one post, we highlighted a revolutionary approach recently approved by the US Food and Drug Administration (FDA) using genetically modified immune cells to fight cancer,... Continue Reading →