Reading time: 5 minutes
Bekah Schulz
The Food and Drug Administration (FDA) is often criticized by patient advocacy groups for taking too much time to approve life-saving drugs. However, the FDA is a difficult situation; if they approve a drug too quickly and it turns out to be unsafe/ineffective, then people are upset. If they take the time necessary to ensure the safety and efficacy of a drug, then people are upset. So let’s take a moment to review what goes into approving a drug, with a special focus on cancer (oncology) drugs.
Approval for First-in-human Studies
Before a drug can be tested in humans, the sponsor (or the group that owns the drug) has to file an Investigational New Drug (IND) application. An IND should show that enough research has been conducted to indicate that a specific drug has a potential benefit in humans and that the drug is safe. The definition of “safe” depends on the drug’s application. With cancer, the difference between the patient getting the drug can be life or death. In these cases, often the drug is not “safe” in a traditional sense. For example, chemotherapy is essentially poison and has severe side effects such as hair loss, vomiting, and possible death. However, if the cancer patient is not treated with chemotherapy, he/she might die. Thus the drug’s benefits are worth the serve side effects. On the other hand, if a drug used to treat type 2 diabetes had these severe side effects, the IND would probably not be approved because there is already FDA-approved treatment (insulin) that will allow the patient to live a long, healthy life. It is the FDA’s responsibility to make a decision on whether the benefits of a drug outweigh the risks.
The IND provides enough information to allow the FDA to make this informed decision, summarized here. An important piece is preclinical data in the form of studies, which are usually performed on at least two different animals to show the safety and efficacy profile of the drug. The IND also includes a section on chemistry manufacturing and controls (CMC). This is a description of how the drug will be produced at a large enough scale to treat humans. A clinical trial protocol is also included in the IND. This covers everything from when patients will be treated to methods for determining if the drug is safe and efficacious. Once all of this information is compiled into a document, the sponsor can submit the IND to the FDA for review.
Clinical Trials
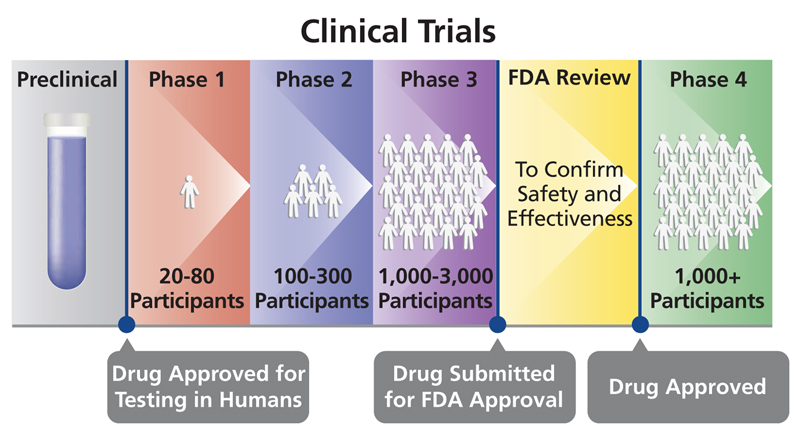
If the FDA does not respond in 30 days, then clinical trials can begin. Clinical trials take an average of seven years to complete. The following are the different stages of clinical trials.
- Phase I: The purpose of this phase is to determine if the drug is safe. In most fields, Phase I studies are done in healthy patients. However, oncology Phase I studies are done in cancer patients because it would not be ethical to treat healthy patients with drugs with known severe side effects. Phase I studies usually involve a small number of patients (less than 100 people). At the end of this phase, a clinical study report (CSR) is submitted to the FDA for review.
- Phase II: If the drug has a promising safety profile as determined by Phase I, Phase II studies can begin. This is the first time the FDA considers the efficacy of a drug; safety will be assessed as well. A larger number (hundreds) of homogeneous patients are enrolled to increase the odds for success. At the end of Phase II studies, a CSR is submitted to the FDA.
- Phase III: If the drug is determined to be safe and efficacious, it will move to Phase III studies. In this phase, thousands of heterogeneous patients are tested to ensure that no side effects were missed in the first two studies. The large number of patients also allows the FDA to better determine the efficacy of the drug. Usually the FDA requires at least two separate Phase III studies. If they are successful, the sponsor will compile all clinical trial data into a New Drug Application (NDA). This will be submitted to the FDA, who will then decide if there is enough information to approve the drug, this can take between ten months to one year.
Postmarket Studies
Often, the FDA’s approval of a drug will be contingent on the sponsor continuing to perform postmarket studies, also called Phase IV studies. For cancer, these studies often look patient survival times, development of resistant to the therapy, and long-term side effects of the therapy. The sponsor must submit posmarket safety update reports (PSURs) on a regular basis. In addition, any unexpected severe adverse events must be reported to the FDA within 15 days of an event occurring. The FDA can take revoke a drug’s approval at any time.
Addressing Patient Concerns
The FDA has created programs to decrease the time it takes to approve life-saving drugs, for instance, Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review. The Fast Track designation is given to drugs to “treat serious conditions and fill an unmet medical need.” Sponsors of these drugs can submit data as they receive it, instead of waiting for completion of a study. Breakthrough Therapies are drugs that demonstrate “substantial improvement over available therapy.” The Accelerated Approval designation is for drugs that treat “serious conditions that fill an unmet medical need to be approved based on a surrogate endpoint.” This designation is often given to cancer therapies, as it can take years to determine if a patient becomes cancer free. Finally, Priority Review ensures that a drug will be reviewed in six months, compared to the normal ten months.
The drug review process might seem cumbersome, but the FDA wants to ensure all approved drugs are safe and efficacious. That said, they have programs to speed the approval of life-saving drugs without compromising the safety of patients. So next time someone complains about the FDA, you can explain that, while there is always room for improvement, there is a lot to consider before approving a new drug.
Edited by Larissa Biggers
Image source: https://aidsinfo.nih.gov/understanding-hiv-aids/glossary/63/approved-drug