Reading time: 6 minutes
Nicole Bowens
Recent advancements within the past decade are making cancer treatments more effective and accessible, revolutionizing the way care is delivered and offering renewed hope to patients. These advancements have been made possible in part by personalized therapies that are tailored for individual patients.
In one newly developed personalized therapy, a type of immune cell, the T cell, is extracted from the patient’s blood and then genetically modified to specifically target and kill cancer cells. The T cell is modified to display receptor proteins that selectively bind to antigen proteins on the surface of cancer cells. These newly created T cells are therefore known as chimeric antigen-receptor T (CAR T) cells. When the receptor of a CAR T cell binds to the targeted antigen on the cancer cell, multiple cell death pathways are activated to kill the cancer cell.
In clinical trials, CAR T has significantly improved survival for patients with hematological cancers, including lymphomas and leukemia. For example, patients with large B-cell lymphoma had a 21% decrease in the risk of death and a 54% decrease in the risk of progression.
In the future, it may also be possible to create “off-the-shelf” CAR T products that are made from healthy people and are therefore immediately available. Researchers are also looking into using this technology to modify other immune cells, including natural killer cells and macrophages.
CAR T-Cell Therapy Process
The patient’s journey through CAR T-cell therapy first begins when the doctor determines if the patient is eligible for the treatment. Patients must be fit enough to receive the therapy and remain stable during the manufacturing process. They must also have adequate caregiver support and remain within a 2-hour driving distance from the CAR T treatment center to monitor and manage any side effects. After T-cell collection, patients are treated with chemotherapy to prepare their bodies to receive the finished CAR T product. Chemotherapy may also be used to reduce cancer progression while the CAR T product is being manufactured. The production process typically takes between 3 to 6 weeks. Once CAR T cells are manufactured and grown, they are reinfused into the patient’s bloodstream.
Inpatient vs Outpatient CAR T
CAR T-cell therapy can cause serious neurological and immunological side effects that may be life threatening. Fortunately, these side effects are manageable and reversible with careful monitoring and prompt treatment. For this reason, CAR T was initially approved only for administration in a hospital where the patient would stay for a couple weeks. However, inpatient administration and management of CAR T-cell therapy comes with high costs to the healthcare system and a significant burden of treatment demands on the patient.
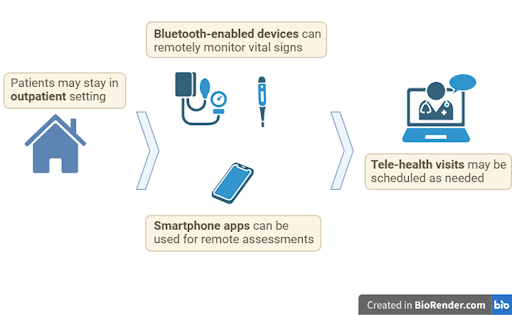
More recently, oncologists have found that CAR T may be administered safely in the outpatient setting without a reduction in the treatment’s effectiveness. However, outpatient CAR T-cell therapy is not appropriate for all patients. Patients who are at low risk for serious side effects and have a caregiver who can give dedicated and continuous around-the-clock care throughout the therapeutic process may greatly benefit from the outpatient setting. In the outpatient setting, patients may stay at their home or at temporary lodging that may be provided by the CAR T care center. The benefits include decreased treatment burden from frequent interruptions for monitoring and medical interventions, increased privacy, and greater freedom to engage in normal activities, like walking around. Additionally, the patients may be more comfortable with continuous support from a loved one.
Innovations in Outpatient CAR T
Considerable innovations in telehealth and remote monitoring, driven in part by the COVID-19 pandemic, have made outpatient CAR T-cell therapy possible. Bluetooth-enabled devices can be used to measure vital signs, such as heart rate, core body temperature, and blood pressure, and electronically transmit this data to the patient’s care team. Such devices include non-invasive wearables for continuous monitoring and patient-operated equipment used in conjunction with smartphone apps. Assessments such as neurological exams may also be completed through phone apps or on a center-provided tablet device. In the case when a regularly scheduled assessment is not completed or when vital signs are abnormal, automated alerts may notify the care team to initiate a prompt tele-health visit. When necessary, members of the patient’s care team may be sent for an in-person visit. Hospitalization may be considered when there are signs or symptoms of severe side effects.
Importance of an Interdisciplinary Team
For outpatient CAR T-cell therapy to be safe, effective, and accessible, patients, caregivers, and an interdisciplinary team of clinicians must collaborate cohesively. The interdisciplinary team may include physicians, advanced practitioners, nurses, and case managers. Nurses and case managers may help to arrange financial accommodations for lodging and food throughout the course of treatment. Advanced practitioners and nurse coordinators work to provide patients and their caregivers with education on what to expect during the course of treatment with CAR T-cell therapy, including the risks and management of potential side effects. Nurses must also carefully educate patients and their caregivers on how to use any necessary devices or other remote monitoring systems, including which vitals to measure, how to measure them, and how often to do so. If severe side effects occur, vigilant monitoring by the caregiver and rapid communication with the care team are essential to mitigate risks.
Future for Outpatient CAR-T
Innovations in the management of CAR T-cell therapy are expected to further enhance the accessibility of this groundbreaking treatment. Recent strategies for early intervention in side effects have shown that intensive remote monitoring may not always be required to ensure safety. Additionally, advances in technology, such as machine learning and AI, hold promise for improving remote monitoring systems by personalizing monitoring and management based on each patient’s unique characteristics, enabling more targeted and efficient care. Machine learning and AI could be used to process data received from remote bluetooth monitoring devices and make predictions on the likelihood of serious side effects. As these innovations continue to evolve, they have the potential to improve patient safety, enhance quality of life, and ensure more personalized and effective care.
Header Image Source: Created by author in Biorender.com
Edited by Hema Saranya Ilamathi
References
Alexander M, Culos K, Roddy J, et al. Chimeric Antigen Receptor T Cell Therapy: A comprehensive review of clinical efficacy, toxicity, and best practices for outpatient administration. Transplant Cell Ther. 2021;27(7):558-570. https://doi.org/10.1016/j.jtct.2021.01.014
Altaf F, Jamil A, Siddique R, Qureshi Z. Efficacy and safety of CAR T-cell therapy in non-Hodgkin lymphoma: A systematic review and meta-analysis. Blood. 2024;144(Suppl 1):6551. https:/doi.org/10.1182/blood-2024-208202.
Baer, B. M., & Long, N. C. (2022). Changing the tune for CAR T-cell therapy: a music city experience in remote patient monitoring. Oncology Issues, 37(6), 20–24. https://doi.org/10.1080/10463356.2022.2124801
Bäckel N, Hort S, Kis T, et al. Elaborating the potential of Artificial Intelligence in automated CAR-T cell manufacturing. Front Mol Med. 2023;3:1250508. https://doi,org/10.3389/fmmed.2023.1250508
Banerjee R, Shah N, Dicker AP. Next-generation implementation of chimeric antigen receptor T-cell therapy using digital health. JCO Clin Cancer Inform. 2021;5:668-678. https://doi.org/10.1200/CCI.21.00023
Beaupierre A, Kahle N, Lundberg R, Patterson A. Educating multidisciplinary care teams, patients, and caregivers on CAR T-cell therapy. J Adv Pract Oncol. 2019;10(Suppl 3):29-40. https://doi.org/10.6004/jadpro.2019.10.4.12
Furqan F, Bhatlapenumarthi V, Dhakal B, et al. Outpatient administration of CAR T-cell therapies using a strategy of no remote monitoring and early CRS intervention. Blood Adv. 2024;8(16):4320-4329. https://doi.org/10.1182/bloodadvances.2024013239
Gatwood K, Mahmoudjafari Z, Baer B, et al. Outpatient CAR T-cell therapy as standard of care: current perspectives and considerations. Clin Hematol Int. 2024;6(2):11-20. Published 2024 Apr 9. https://doi.org/10.46989/001c.115793
Hansen DK, Liu YH, Ranjan S, et al. The Impact of outpatient versus inpatient administration of CAR-T therapies on clinical, economic, and humanistic outcomes in patients with hematological cancer: a systematic literature review. Cancers (Basel). 2023;15(24):5746. https://doi.org/10.3390/cancers15245746
Llaurador GA, Heslop HE, Steffin DH. Moving CAR-Ts to the outpatient clinic. Br J Haematol. 2023;203(4):507-508. https://doi.org/10.1111/bjh.19129
Mohty R, Lazaryan A. “Off-The-Shelf” allogeneic chimeric antigen receptor T-cell therapy for B-cell malignancies: current clinical evidence and challenges. Front Oncol. 2024;14:1433432. http://doi.org/10.3389/fonc.2024.1433432
National Cancer Institute. CAR T-cell therapy. Accessed February 25, 2025. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy
Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41(1):119. https://doi.org/10.1186/s13046-022-02327-z

Leave a comment