Reading time: 3 minutes
Alejandra Canales
Finding treatments for lung cancer is hard because, simply-put, cancerous cells are extremely stubborn, and patients with non-small cell lung cancer can often acquire resistance to a treatment regimen.
Researchers have been exploring the feasibility of “liquid biopsies,” whereby a non-invasive blood sample could be used to obtain information about a patient’s cancer rather than an invasive surgical biopsy. Now, scientists are demonstrating the clinical utility of liquid biopsies in monitoring responses in a notoriously treatment-resistant population of non-small cell lung cancer patients.
Genetic testing has become increasingly more common in cancer patients. Scientists have known about cancer-causing mutations in the ALK gene for more than a decade, and non-small cell lung cancer patients are the largest patient group that carries ALK mutations. This gene encodes a protein that can activate many of the cellular signaling events that lead to uncontrolled growth and division of cancerous cells, so a critical aspect of care for patients with these mutations includes testing for any genetic ALK genetic variants and treating their cancer with drugs called tyrosine kinase inhibitors to block the activity of the ALK protein.
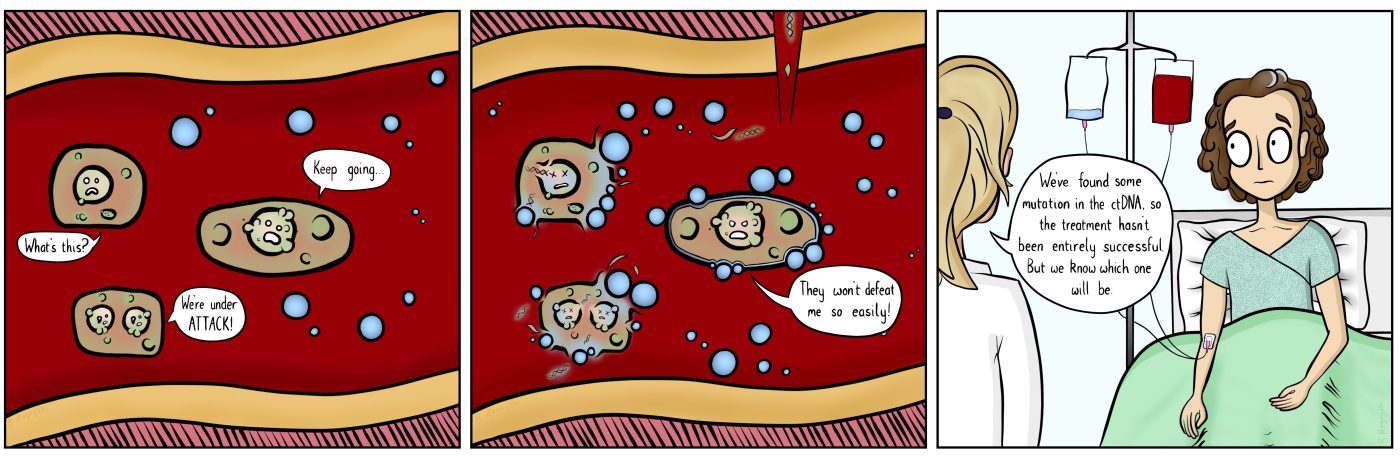
However, many patients still end up becoming resistant to those drugs after a couple of years. The tumor cells either figure out alternative ways to activate cellular signaling or evolve new mutations in the ALK gene. These can be point mutations, deletions, or even large rearrangements that lead to parts of the ALK gene with fusing with other genes, and developing methods sensitive enough to detect all these types of changes to the ALK gene has been challenging.
Advances in DNA sequencing have allowed for more rapid and precise detection of ALK gene rearrangements from patient blood samples. Tumor DNA circulates in the blood at low concentrations, too low for traditional sequencing methods. These new methods enrich for the amount of tumor DNA present by using short sequences called “adapters” to amplify the DNA. The adapters also serve as unique identifiers for each region of DNA amplified. Using this approach, researchers at the Massachusetts General Hospital in Boston showed that they could detect changes in the ALK gene in patients.
Building on this work, a recent study led by Dr. Leora Horn from Vanderbilt University, researchers focused on patients enrolled in a phase I/II clinical trial with the tyrosine kinase inhibitor, ensartinib. Since patients taking this medication have not reported serious side effects, clinicians are interested in its use as a first-line therapy. They also want to know whether patients develop resistance to the drug. The researchers collected liquid biopsies from 76 patients that had tested positive for ALK mutations. They found that these sequencing methods enabled them to identify both mutations in the ALK gene as well as ALK gene fusions. Since they also had access to tumor biopsy samples from a subset of the patients, the researchers were also able to compare their sequencing results across different sample types. They found that their results from blood samples and tumor samples matched in 91 percent of patients with an ALK gene fusion. Collecting this sequencing data prior to treatment allowed Horn and her colleagues to identify the patients who might be more responsive based on their gene mutations.
The researchers also collected longitudinal plasma samples for 11 patients in their cohort to monitor how the patients were responding to the drug treatment. One of the deleterious mutations they identified in from the plasma sample was present before progression of the cancer was detected by radiography.
While the results of studies like this still need further validation, these initial results show that “liquid biopsies” might provide a useful way to monitor a cancer patient’s progress.
Edited by Rachel Cherney
Works discussed:
- Horn, L., Whisenant, J.G., Wakelee, H., Reckamp, K.L., Qiao, H., Leal, T.A.,..Lovly, C.M. (2019). Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. Journal of Thoracic Oncology, 14(11): 1901-1911, doi: doi.org/10.1016/j.jtho.2019.08.003.
- Raymond, C. K., Hernandez, J., Karr, R., Hill, K., & Li, M. (2017). Collection of cell-free DNA for genomic analysis of solid tumors in a clinical laboratory setting. PloS one, 12(4), e0176241. doi:10.1371/journal.pone.0176241
- Horn, L., Infante, J. R., Reckamp, K. L., Blumenschein, G. R., Leal, T. A., Waqar, S. N., … Wakelee, H. A. (2018). Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(12), 2771–2779. doi:10.1158/1078-0432.CCR-17-2398
- Dagogo-Jack, I., Brannon, A. R., Ferris, L. A., Campbell, C. D., Lin, J. J., Schultz, K. R., … Gainor, J. F. (2018). Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors through Longitudinal Analysis of Circulating Tumor DNA. JCO precision oncology, 2018, 10.1200/PO.17.00160. doi: 10.1200/PO.17.00160
- McCoach, C. E., Blakely, C. M., Banks, K. C., Levy, B., Chue, B. M., Raymond, V. M., … Doebele, R. C. (2018). Clinical Utility of Cell-Free DNA for the Detection of ALK Fusions and Genomic Mechanisms of ALK Inhibitor Resistance in Non-Small Cell Lung Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(12), 2758–2770. doi:10.1158/1078-0432.CCR-17-2588
Header Image: Beth Rogoyski. See Beth’s original article for OncoBites, on liquid biopsies, from August 2018. ctDNA stands for circulating tumor DNA.

Leave a comment