Reading time: 5 minutes
Hema Saranya Ilamathi
It’s seven o’clock in the morning! A bright light hits my face, and I awaken before the alarm clock starts buzzing. Did you know humans have an inbuilt biological clock known as the circadian rhythm? Light stimulates this 24-hour central clock in the brain. In general, photoreceptors in the eye detect light and activate the suprachiasmatic nucleus in the brain. This specialized structure in the brain secretes hormones and signal molecules (e.g., neuropeptides and metabolites) that activate and synchronize the peripheral clock in the rest of the organs. However, the peripheral clock still functions independently of the stimuli from the central clock. The circadian rhythm is influenced by time signals known as Zeitgebers, which include light and darkness, feeding and fasting, exercise and rest, social interactions, and temperature. Changes in this rhythmic pattern may raise the risk of chronic diseases such as cancer.
Biological clock
About 15 circadian clock proteins work in harmony to maintain rhythmicity by regulating different biological activities in the body. BMAL1-CLOCK is the master regulator of the circadian clock. These protein complexes regulate the expression of the rest of the clock genes. Generally, circadian proteins are controlled by a feedback loop in which an increase in certain circadian rhythm-related proteins blocks the master protein, BMAL1-CLOCK. This feedback loop creates 24-hour rhythmicity and helps to restart a new 24-hour clock. For instance, BMAL1-CLOCK protein levels rise during the day, which increases the expression of circadian genes, PER-CRY. By the end of the day, a rise in PER-CRY protein levels inhibits BMAL1-CLOCK protein function. This, in turn, prevents an increase in PER-CRY levels, which are further degraded during the night, freeing up BMAL1-CLOCK for a new cycle. Thus, circadian protein levels are coordinated with the light cycle. This rhythmic pattern is essential for the regulation of various cellular functions.
Circadian rhythm and its role in cancer
Many studies have attempted to link an altered sleep/wake cycle on circadian rhythms with an increased risk of developing cancer. According to these studies, the World Health Organization (WHO) has considered night shift work as a possible carcinogen (1). Other studies, however, have found no link between night shift work and cancer risk (2). Further studies must be conducted with a detailed set of guidelines to better understand the relationship between nocturnal work and cancer predisposition. For instance, a uniform set of criteria must be established to describe changes in sleep/wake habits. Additional quantifiable factors (e.g., circadian rhythm-related genes) must also be defined to detect rhythmicity alteration.
The impact of circadian rhythm on cancer development is still under debate. Nonetheless, growing evidence indicates that the circadian rhythm may play a role in cancer progression. Circadian rhythms regulate cellular processes such as cellular multiplication, DNA repair, immune response, and cellular metabolism, all of which are impaired in cancer cells. However, depending on the cancer cell type, these cellular processes are differentially regulated by the circadian genes. This limits extrapolating a direct correlation between the impact of circadian rhythm on these cellular processes and cancer.
Chronotherapy
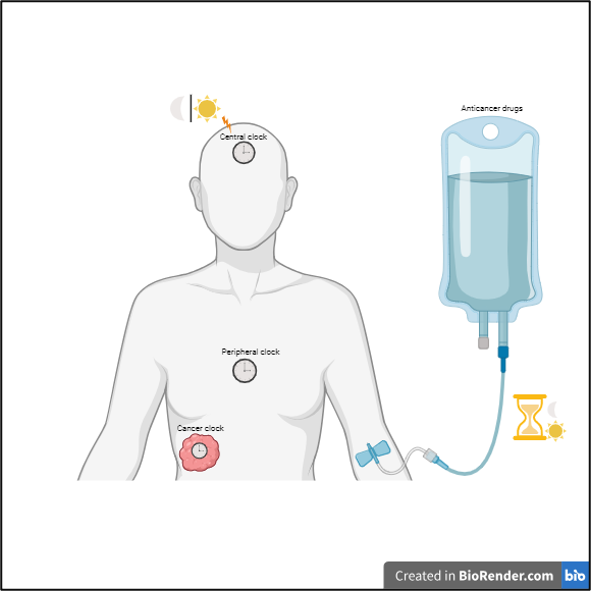
Given that the circadian rhythm regulates the function of cells, it is plausible that drugs that target these distinct cellular processes could be impacted by this internal biological clock. Chronotherapy, in which anticancer drugs are dosed based on the person’s biological clock, is gaining popularity among cancer treatments. It begins with diagnosing whether patients have an intact or disrupted biological clock. This information is crucial for chronotherapy as cancer treatments are influenced by the biological clock of the patient. For instance, certain treatments have better outcomes when the circadian rhythm is intact, whereas the opposite is true in other cases. Given this, a combined approach involving biological clock activation/suppression and timely dosing of anticancer drugs may improve treatment outcomes.
Numerous pieces of evidence highlight the potential of chronotherapy in cancer treatment. The most well-known example is the anticancer medication oxaliplatin, which was once removed from clinical trials because of toxicity. However, administering Oxaliplatin in the evening (16h) showed promising results against colorectal cancers. In addition to improving treatment response, timed dosing of the drug decreases harmful side effects. For example, treating ovarian cancer patients with doxorubicin in the morning (6h) and cisplatin in the evening (16-20h) has been shown to reduce drug toxicity. Like chemotherapeutic drugs, immunotherapeutic agents induce an effective antitumor immune response when administered at the appropriate time. For instance, administering antibodies targeting immune checkpoints (proteins that suppress the antitumor immune response) in the morning has shown an increase in survival in patients with advanced melanoma.
Challenges of Chronotherapy in Standard Healthcare
Although chronotherapy is still in its infancy as a cancer treatment modality, clinical trials have shown encouraging results. Chronotherapy is a personalized treatment approach, and its outcome is influenced by different factors. Notable among these are gender, age, chronotype (naturally preferred time to sleep/wake), cancer type, the condition/status of the circadian rhythm in patients, the impact of circadian rhythm on drug efficacy, the drug effect on the patient’s circadian rhythm, and other health complications in patients. Considering these factors during clinical trials might help to bring chronotherapy into the standard cancer treatment regimen. Furthermore, non-invasive techniques for measuring the circadian rhythmic pattern in cancer cells, in addition to the internal body clock (central and peripheral clock), could bring chronotherapy into the spotlight for cancer treatment.
Edited by Ana Isabel Castillo Orozco
References
1. Straif, K., Baan, R., Grosse, Y., Secretan, B., El Ghissassi, F., Bouvard, V., Altieri, A., Benbrahim-Tallaa, L., Cogliano, V., and Group, W.H.O.I.A.F.R.o.C.M.W. (2007). Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8, 1065-1066. 10.1016/S1470-2045(07)70373-X.
2. Sancar, A., and Van Gelder, R.N. (2021). Clocks, cancer, and chronochemotherapy. Science 371. 10.1126/science.abb0738.
3. Zhou, L., Zhang, Z., Nice, E., Huang, C., Zhang, W., and Tang, Y. (2022). Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Hematol Oncol 15, 21. 10.1186/s13045-022-01238-y.
4. Amiama-Roig, A., Verdugo-Sivianes, E.M., Carnero, A., and Blanco, J.R. (2022). Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers (Basel) 14. 10.3390/cancers14205071.

Leave a comment