Reading time: 4 minutes
Susy Prieto Huarcaya, PhD
For families facing diffuse midline glioma, time often feels painfully short and options even shorter. That’s why the FDA’s approval of dordaviprone (Modeyso™) marks a historic turning point—the first therapy for gliomas carrying the H3K27M mutation.
What Are Diffuse Midline Gliomas?
Diffuse midline glioma (DMG) is a rare but extremely aggressive brain tumor that mainly affects children and young adults. These tumors arise from glial cells—the “support cells” of the brain—and grow along midline structures like the thalamus, brainstem, or even the spinal cord. Because these areas control vital functions such as movement, breathing, and heart rate, surgery is impossible.
Radiation therapy, the current standard treatment, can slow tumor growth for a short time, but it cannot cure the disease. As a result, DMG has long been one of the most devastating diagnoses in pediatric oncology.
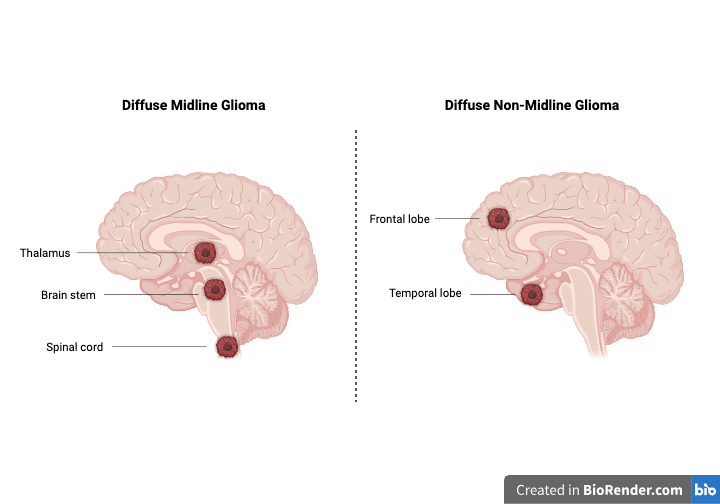
What Is the H3K27M Mutation?
Much like yarn wound around a spool, DNA is neatly wrapped around tiny protein spools called histones to stay organized inside each of our cells. Specific chemical modifications on the histones make the DNA “tighter” or “looser” around them, regulating gene expression. H3K27 refers to the aminoacid Lysine 27 (K27) in histone 3 (H3). At this position, histone 3 can get two different chemical marks— trimethylation (m3) or acetylation—that stop or activate gene expression.
The H3K27M mutation affects histone H3, removing the trimethylation mark (H3K27me3) that acts like a stop signal for certain genes. Without it, gene activity becomes chaotic, driving the uncontrolled growth of cancer cells. Tumors with this mutation tend to grow faster and have worse outcomes than those without it, making a targeted treatment even more urgent.
In August 2025, the FDA approved dordaviprone (Modeyso™) as the first and only treatment specifically for diffuse midline gliomas carrying the H3K27M mutation.
How Does Dordaviprone Work?
Dordaviprone is an oral small-molecule drug that can cross the blood–brain barrier, allowing it to reach the tumor where it grows.
It works by targeting two key vulnerabilities inside cancer cells:
- Dopamine Receptor D2 (DRD2): DMG tumors produce too much DRD2, a protein on the cell surface that fuels cancer growth through pathways like Akt and RAS. Dordaviprone blocks DRD2, cutting off this growth signal.
- Mitochondrial Protease ClpP: Cancer cells rely on ClpP, which gets rid of damaged proteins, to keep their mitochondria—their power plants—working properly. Dordaviprone overactivates ClpP, disrupting energy production and triggering tumor cell death.
In laboratory studies with patient samples, dordaviprone increased levels of a particular metabolite, L-2-hydroxyglutarate, which blocks the removal of H3K27me3—the very chemical mark lost due to the H3K27M mutation. In other words, it helps reverse the mutation’s harmful effects.
Dordaviprone in Diffuse Midline Gliomas (DMG)
A study by Arrillaga-Romany et al. (2024) evaluated dordaviprone in 50 patients (4 children and 46 adults) with recurrent H3K27M-mutant DMG. Participants took 625 mg of dordaviprone (in capsules) weekly and underwent regular MRI scans.
Tumor shrinkage became detectable around 8 months after starting treatment. But even more impressive, patients reported improved quality of life in just 3.5 months. These improvements matched a 50% reduction in corticosteroid use.
People with DMG often take corticosteroids to treat tumor-related symptoms like brain swelling. Unfortunately, their prolonged usage, especially at high doses, can be toxic. Low corticosteroid intake shows that the patients experienced fewer DMG-related symptoms. This early drop in inflammation suggests that dordaviprone starts working on the tumor’s root problems sooner than expected.
For a disease that typically progresses rapidly and has a lethal prognosis, such tangible clinical improvement brings a powerful message of hope.
Beyond the Midline: Dordaviprone for Non-Midline Gliomas
Interestingly, dordaviprone also showed benefits in non-midline gliomas carrying the same H3K27M mutation. Odia et al. (2024) studied five patients with tumors in the frontal, temporal, or parietal lobes.
Clear tumor shrinkage was observed in multiple patients. Impressively, one adult patient returned to normal everyday activity with no need for corticosteroids for 41 months, while a child experienced improved mobility and alertness.
These findings confirm that it is the mutation, not the tumor’s location, that predicts response to dordaviprone.
What Comes Next?
A major Phase 3 clinical trial (NCT05580562) is now underway, testing whether dordaviprone can extend survival and delay tumor progression in newly diagnosed H3K27M-positive patients after radiation therapy. Results are expected in mid-2026 and may reshape the entire treatment landscape for DMG.
Header Image Source: File:Palliative Care Options for a Young Adult Patient with a Diffuse Intrinsic Pontine Glioma – Fig. 3 (cropped).png – Wikimedia Commons
Second Image Source: Created by Susy Prieto Huarcaya with Biorender.
Edited by Preeti Prangya Panda
References
Akdemir EY, Odia Y, Hall MD, Mehta MP, Kotecha R. An Update on H3K27M-altered Diffuse Midline Glioma: Diagnostic and Therapeutic Challenges in Clinical Practice. Pract Radiat Oncol. 2024;14(5):443-451. doi:10.1016/j.prro.2024.04.013
Arrillaga-Romany I, Gardner SL, Odia Y, et al. ONC201 (Dordaviprone) in Recurrent H3 K27M-Mutant Diffuse Midline Glioma. J Clin Oncol. 2024;42(13):1542-1552. doi:10.1200/JCO.23.01134
Odia Y, Hall MD, Cloughesy TF, et al. Selective DRD2 antagonist and ClpP agonist ONC201 in a recurrent non-midline H3 K27M-mutant glioma cohort. Neuro Oncol. 2024;26(Supplement_2):S165-S172. doi:10.1093/neuonc/noae021

Leave a comment