Reading time: 13 minutes (special: includes a pre- and post-article quiz activity!)
Néstor Zumaya
Activity: Choose the right answer based on your current knowledge. You’ll have the chance to retest yourself after reading the entire article.
- Current reliable biomarkers for treatment selection in R/R DLCBL
- No reliable biomarkers available. Response to therapy is the most powerful prognostic indicator
- CD19 and CD20
- CD19 only
- CD4
- Cytokine release syndrome (CRS) from epcoritamab (and other agents) can be mitigated using the following approach:
- Initial full dose and holding while monitoring for CRS
- Two step-up doses before the first full dose
- Full dose divided in two
- IV agents only
- For relapse following treatment with CAR-T or auto-SCT, these therapies are likely to be more efficacious and better tolerated for most patients
- Already approved therapies used as second or third line therapies
- Combination therapies only
- Synergy bispecific agents
- Novel therapies
- The benchmark of outcomes for patients with relapsed disease (R/R DLBCL) is a median overall survival of:
- 5 years
- 6.3 months
- 2.2 years
- 12 months
- Potential relapse mitigation therapies after CAR-T include the following:
- Already approved therapies for R/R DLBCL
- Auto-SCT
- Bispecific agents
- Cytotoxic chemotherapy only
- One of the main mechanisms of single agent bispecific therapy resistance is the following:
- CD19 overexpression
- T-cell overproduction
- T-cell exhaustion
- CD20 underexpression
- Every patient with suspected relapse should be evaluated first for:
- Clinical confirmation and biomarker assessment
- Clinical confirmation CD19 and CD20 expression
- Confirmation through biopsy
- TEAEs
- Cytotoxic chemotherapy-free alternatives offer the following benefit:
- Less serious AE profile
- Mainly hematologic AE profile
- Synergy through T-cell depletion
- Prevent exhaustion of T-cells
Introduction
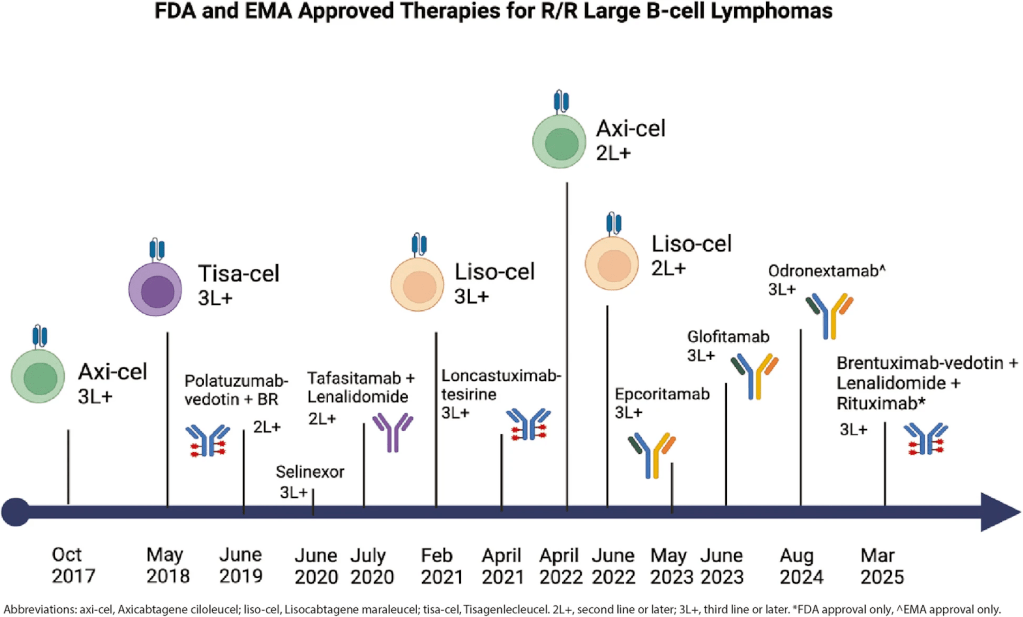
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin lymphoma. It can be cured in 60-70% of patients with frontline immunotherapy. No overall survival benefit has been seen with polatuzumab-vedotin in combination with rituximab, cyclophosphamide, doxorubicin and prednisone for high-risk patients (IPI score of 2 or greater) who develop primary refractory disease or early relapse compared to rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). Treatment remains challenging for the proportion of patients (30–40%) that relapse after treatment with frontline immunochemotherapy.
Survival for patients with refractory disease has been particularly poor, with the benchmark for outcomes for patients with relapsed disease (R/R DLBCL) being a median overall survival of 6.3 months from the start of salvage therapy, which is derived from the multicenter retrospective study Scholar-1, published in 2017. This study demonstrated the need for more effective therapies in the context of R/R DLBCL.
Previously, over 80% of patients failed to respond sufficiently to second-line chemotherapy or were ineligible to receive autologous stem cell transplant (auto-SCT). Within the last 10 years, the treatment for patients with DLBCL went from platinum-based therapies to the development of CAR-T therapy and other targeted and combination treatment options. Despite these advancements, there is no clear evidence on sequencing and optimal treatment selection, and the majority of patients relapse within 6 months of initiation.
Current therapies
Bispecific antibodies (BsAbs)
Bispecific antibodies activate effector T-cell response against malignant lymphoma B-cells. These are developed to target more than one antigen, unlike CAR-T. Some of the benefits of BsAbs include longer half-life and less neurotoxicity. Current approved BsAbs include epcoritamab, glofitamab, and odronextamab.
Antibody-drug conjugates (ADCs)
ADCs work by delivering a cytotoxic molecule intracellularly, which limits their systemic toxicity. There are currently three approved therapies: polatuzumab-vedotin, loncastuximab-tesirine, and brentuximab-vedotin. Some combinations of polatuzumab have achieved an OS of 12.4 months. The anti-CD19 IgG1 antibody loncastuximab has a lower OS of 4.6 months in primary refractory disease. The combination of brentuximab + lenalidomide + rituximab has been proven to have higher efficacy outcomes compared to rituximab alone, with an OS of 13.8 months vs. 8.5 months.
Small molecule inhibitors
The approved selective inhibitor of XPO1-mediated nuclear export selinixor works by reducing MYC and BCL2 oncogenes. The treatment emergent adverse events (TEAEs) of selinixor have led to dose modification in 70% of patients and discontinuation in 17%. Given its low tolerability, it is not a recommended therapy.
Non-cellular immune therapies
The combination of tafasitamab and lenalidomide was studied in patients that had been ineligible for auto-SCT or in patients with 1–3 prior lines of therapy. The median duration of response was 43.9 months with a median progression-free survival of 11.6 months, and a median OS of 33.5 months. The most common TEAE was neutropenia, and the most common severe AE was neutropenia. After 35 months, the objective response rate (ORR) was 57.5% and the complete response (CR) rate was 40%, and, among those with CR, the median OS has not been reached, with a 24-month OS of 90.6%.
Autologous stem cell transplant (auto-SCT)
CAR-T and auto-SCT represent curative treatment options, and determining eligibility of patients is the first step for patients with R/R DLBCL. Eligibility for auto-SCT is primarily determined by adequate cardiac, pulmonary, renal and hepatic function, and is measured by the hematopoietic cell transplantation-specific comorbidity index (HCT-CI); although developed to assess risk after allogeneic transplantation, it can also predict non-relapse mortality after auto-SCT.
The current standard of care still is platinum-based chemotherapy followed by auto-SCT in cases that achieve chemosensitivity. This practice leads to a cure in close to 20% of patients with relapse. Approximately 40-50% of these patients will experience durable remission.
Chimeric antigen receptor T-cell therapy (CAR-T)
This is a type of therapy that uses genetically modified T-cells that express a chimeric antigen receptor, an engineered receptor with an extracellular tumor antigen binding domain, and an intracellular T-cell signaling domain. The destruction of malignant B-cells follows the activation of T-cell activation by CAR-T cells. Anti-CD19 CAR-T therapies had been approved as second line treatment in the R/R DLBCL setting (tisa-cel, axi-cel and liso-cel), and there had been more recent approvals in the second line setting (axi-cel and liso-cel).
CAR-T is a reasonable choice for patients experiencing relapse when they are not eligible for auto-SCT or if they do not reach CR after second line platinum-based chemotherapy.
Table 1. Approved CAR therapies for R/R DLBCL
| Treatment | Phase | ORR (%) | CR (%) | PFS estimate | Median PFS (months) | Median OS (months) |
| Second-line | ||||||
| axi-cel | 3 | 83 | 66 | 2 year, 46% | 14.7 | Not reached |
| liso-cel | 3 | 86 | 66 | 36 months, 50.9% | 10.1 | Not reached |
| Third-line | ||||||
| axi-cel | 2 | 83 | 59 | 15 months, 41% | 5.8 | Not reached |
| liso-cel | 1/2 | 73 | 53 | 12 months, 44.1% | 6.8 | 21.1 |
| tisa-cel | 2 | 52 | 40 | 12 months, 40% | Not reported | 8.3 |
CR = complete response rate; ORR = overall response rate; OS = overall survival; PFS = progression free survival. axi-cel = axicabtagene ciloleucel, liso-cel = lisocabtagene maraleucel; tisa-cel = tisagenlecleucel.
Eligibility
CAR-T does not share the same criteria as auto-SCT. This therapy increases the cure for older patients without comorbidities. It also hasn’t increased risk of infections or rates of cytokine-release syndrome (CRS) or immune effector cell associated neurotoxicity syndrome (ICANS). The recommendation still is to have a CD4 count above 200 and undetectable viral load, despite studies showing that CAR-T manufacturing is maintained with lower CD4 counts.
Second line therapy
Compared to standard of care (SOC) of platinum-based therapies followed by auto-SCT in patients with early relapsing (relapse in less than 12 months after frontline chemoimmunotherapy) or refractory DLBCL, CD19 CAR-T showed benefits over auto-SCT in event-free survival (EFS) and OS in the second line setting. With a median follow-up of 24.9 months, the median EFS was 8.3 months with axi-cel vs. 2.0 months with auto-SCT, with 4 year OS after additional 47.2 months reaching 54.6% (axi-cel) and 46% (auto-SCT) in the ZUMA-7 study. Other two studies have demonstrated similar benefits of CAR-T over auto-SCT.
Relapse mitigation strategies
Many of these strategies are currently on development, and these focus on those patients that are slow at achieving an early CR. There are also trials targeted to patients with PR to prevent overt relapse due to the high risk of relapse after CAR-T within the first 6 months after therapy. The main mechanism of these strategies is synergism between therapies. Therapies already approved for the R/R setting are also being tested for relapse mitigation after CAR-T.
Relapse post CAR-T
Between 50% and 60% of patients do not respond or eventually relapse after CAR-T therapy. Confirmation of relapse is done through biopsy, followed by an assessment of CD19 and CD20, although the clinical threshold of CD19 expression necessary for treatment response has not been established. It is recommended that patients be enrolled in clinical trials.
Therapies under investigation
Bispecific antibodies
Mosunetuzumab (CD20xCD3) is an antibody with near-human similarity that has been developed in both IV and subcutaneous (SC) formulations. It is usually administered in a step-up regimen to avoid CRS. Both presentations showed similar CR (IV, 19.4%; SC, 22.2%), with a study showing a median durable response of 20.4 months. Mosunetuzumab has not been approved as a single agent for R/R DLBC.
Planotamab (CD20xCD3) is also being evaluated in both IV and SC formulations; it also requires a step-up approach to prevent CRS and neurotoxicity. Dose-escalation studies are still ongoing.
AZD0486 (CD19xCD3) has a unique low C3 affinity mechanism to reduce cytokine release upon T-cell activation that also preserves effective T-cell cytotoxicity against malignant B cells. Higher response rates were seen in those receiving the target dose and those CAR-T naïve.
FS118 simultaneously targets PD-L1 and LAG-3, two immune checkpoints. The efficacy of a double target therapeutic vs. a single PD-L1 is still in question, and no checkpoint inhibitor has been approved for R/R DLBCL.
Antibody-drug conjugates
Zilovertamab-vedotin has been evaluated for R/R DLBCL. It is a ROR1-targeting antibody-drug conjugate that has been tested as third line therapy for patients not eligible for auto-SCT or CAR-T. It has shown a PFS of 2.5 months and a median OS of 10.6 months.
Non-cellular immune therapies
Iberdomide and golcadomide are two cereblon E3 ligase (CELMoD) therapies that have shown superior cytotoxic activity and generation of the closed conformation of the cereblon complex. Both drugs have a primarily hematologic adverse event profile.
Magrolimab is an anti-CD47 antibody that takes advantage of the upregulation of CD47 in certain lymphomas, including DLBCL. When combined with rituximab, magrolimab has shown an ORR of 24% and a CR of 12% for a median follow-up of 7.9 months in the context of R/R DLBCL.
Combination therapies in relapse
Currently, several studies are evaluating combinations of agents such as bispecific antibodies and tafasitamab-lenalidomide, cytotoxic agents, as well as antibody-drug conjugates polatuzumab-vedotin and loncastuximab-tesirine. The preliminary results are promising. It remains to be seen if these agents benefit from a sequential vs. simultaneous approach.
The combination of bispecific antibodies and cytotoxic agents seems to be emerging as a real alternative, despite initial skepticism. Preclinical studies have demonstrated that BsABs can still be activated even in presence of a low number of T-cells, enabling combination therapies. Several combinations are currently being investigated, including glofitamab in combination with rituximab, ifosphamide, carboplatin, and etoposide.
The synergism between bispecific antibodies and ADCs is also being investigated, since the combination of approved single agents has the potential to broaden the therapeutic options for those who are no eligible for CAR-T or have relapsed after CAR-T. For this, mosunetuzumab and glofitamab have been added to polatuzumab for patients with R/R DLBCL without new safety signals observed. Similarly, combinations of active therapies and novel agents are currently in research for R/R DLCBL, such as monoclonal antibodies and PD-L1 inhibitors, Bruton tyrosine kinase inhibitors (BTKi), and other bispecific antibodies and CELMoDs.
Glofitamab is being investigated alongside englumafusp alpha as a potential bispecific antibody combination. Being a chemotherapy-free combination, the aim is that it helps to increase T-cell activity, as well as the antitumor activity of glofitamab. Data showed that 9 out of 10 patients experienced TEAEs. Since T-cell exhaustion is one of the main mechanisms of resistance to single-agent bispecific therapy, this combination is encouraging.
Table 2. Single-agent therapies in investigation for R/R DLBCL
| Treatment | Phase |
| Bispecific antibodies | |
| Mosunetuzumab | 1/2 |
| AZD0486 | 1 |
| Plamotamab | 1 |
| Non-cellular, immune-based or antibody-based therapies | |
| Zilovertamab-vedotin | 2 |
| Magrolimab (± Rituximab) | 1/2 |
| Golcadomide (±rituximab) | 1/2 |
| Iberomide | 1/2 |
Table 3. Combination therapies in investigation for R/R DLBCL
| Treatment | Phase |
| Epcoritamab + R-DHAX | 1/2 |
| Epcoritamab + GemOx | 1/2 |
| Mosunetuzumab + polatuzumab vs. R-Polatuzumab | 2 |
| Glofitamab + Polatuzumab | 1b/2 |
| Glofitamab + R-ICE | 1b |
| Glofitamab + GemOx vs. GemOx | 3 |
| Loncastuximab + Venetoclax | 1/2 |
| Zanubrutinib + Lenalidomide | 1 |
| Gloftamab + Englumafusp alpha | 1/2 |
| VIPOR | 1b/2 |
Management of patients with R/R DLBCL
More than 30% of DLBCL patients experience relapse after first-line therapy. The current therapeutic options offer improvements over cytotoxic agents and provide CR with durable remission for over 2 years; this had not been seen with chemotherapy based on cytotoxic agents. The main caveat is that none of these therapies work for every patient, highlighting the need for biomarker assessments for both response and resistance.
Every patient with suspected relapse should receive confirmation through biopsy, since other conditions like infections or sarcoidosis present themselves as lymphoma. It is also essential to evaluate the expression of CD19 and CD20 as part of the treatment selection, especially considering how many of the recently approved therapies target one of these surface molecules.
Given the molecular heterogeneity of DLBCL, next generation sequencing techniques may provide insights into disease progression pathways, including identification of risk factors that would be useful to personalize therapy. Any patient with suspected primary refractory disease should be evaluated for eligibility for auto-SCR or CAR-T. For those that are not eligible for auto-SCR or CAR-T, or that have relapsed after these options, novel therapies are likely to be more efficacious, as well as better tolerated.
Time to test your knowledge. Choose the right answer based on the information on this article.
- Current reliable biomarkers for treatment selection in R/R DLCBL
- No reliable biomarkers available. Response to therapy is the most powerful prognostic indicator
- CD19 and CD20
- CD19 only
- CD4
- Cytokine release syndrome (CRS) from epcoritamab (and other agents) can be mitigated using the following approach:
- Initial full dose and holding while monitoring for CRS
- Two step-up doses before the first full dose
- Full dose divided in two
- IV agents only
- For relapse following treatment with CAR-T or auto-SCT, these therapies are likely to be more efficacious and better tolerated for most patients
- Already approved therapies used as second or third line therapies
- Combination therapies only
- Synergy bispecific agents
- Novel therapies
- The benchmark of outcomes for patients with relapsed disease (R/R DLBCL) is a median overall survival of:
- 5 years
- 6.3 months
- 2.2 years
- 12 months
- Potential relapse mitigation therapies after CAR-T include the following:
- Already approved therapies for R/R DLBCL
- Auto-SCT
- Bispecific agents
- Cytotoxic chemotherapy only
- One of the main mechanisms of single agent bispecific therapy resistance is the following:
- CD19 overexpression
- T-cell overproduction
- T-cell exhaustion
- CD20 underexpression
- Every patient with suspected relapse should be evaluated first for:
- Clinical confirmation and biomarker assessment
- Clinical confirmation CD19 and CD20 expression
- Confirmation through biopsy
- TEAEs
- Cytotoxic chemotherapy-free alternatives offer the following benefit:
- Less serious AE profile
- Mainly hematologic AE profile
- Synergy through T-cell depletion
- Prevent exhaustion of T-cells
Answers
1, a; 2, b; 3, d; 4, b; 5, a; 6, c; 7, c; 8, d.
Header Image Source: Created by Jessica Desamero with Canva.com
Second Image Source: Bock AM, Narendranath Epperla. Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: Recent advances and emerging therapies. Journal of Hematology & Oncology. 2025 Jul 1;18(1). https://pmc.ncbi.nlm.nih.gov/articles/PMC12217534/
Edited by Zoya Ahmed
Bibliography
Bock AM, Narendranath Epperla. Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: Recent advances and emerging therapies. Journal of Hematology & Oncology. 2025 Jul 1;18(1). https://doi.org/10.1186/s13045-025-01702-5.
Hutchings M, Dickinson MJ, Gritti G, et al. Englumafusp Alfa (CD19-4-1BBL) Combined with Glofitamab Is Safe and Efficacious in Patients with r/r B-NHL: Extended Follow up Analysis of the Dose-Escalation Part of Phase 1 Trial BP41072. Blood. 2024;144(Supplement 1):990–990. https://doi. org/10.1182/ blood-2024-200096.
Kurtz DM, Scherer F, Jin MC, et al. Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2018;36(28):2845–53. https://doi. org/10.1200/jco.2018.78.5246.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/ 10.1056/ NEJMoa1707447.
Plaks V, Rossi JM, Chou J, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138(12):1081–5. https://doi. org/10.1182/blood.2021010930.


Leave a comment