Keyword: Preclinical oncology imaging
Reading time: 6 minutes
Bethany Cooper
Introduction
With almost 10 million deaths from cancer in 2020, it is the leading cause of death globally, accounting for nearly one in every six deaths. Given the scale and burden of disease, it is no surprise that oncology trials make up a significant proportion of medical research and clinical development. Despite its global R&D focus, around 97% of oncology clinical trials fail to receive FDA approval. This figure reflects the complexity of cancer biology, the challenges of oncology trial design, and the high regulatory bar for safety and efficacy, which must be met for novel therapies to reach real-world patients. With this in mind, the need for early, non-invasive preclinical insight into drug-target interactions and tumor response is becoming increasingly important.
Preclinical insights into molecular and disease mechanisms are paving the way for the development of drugs that specifically target underlying cancer mechanisms. Researchers are gaining a much-needed understanding of how the disease behaves at a biological level, meaning they can design potential therapies that act on the underlying mechanism of the disease. This pre-clinical feedback, driven by preclinical oncology imaging, has a clear role in reducing the oncology trial failure rate, acting as a translational bridge to effective and coveted therapeutic interventions. This article will discuss the role of preclinical oncology imaging in accelerating cancer drug development.
From screening to lead selection
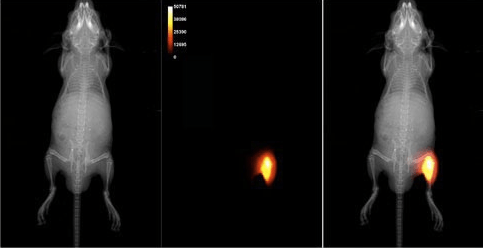
Preclinical imaging is the visualization of living animals for research purposes, allowing researchers to analyze anatomical, physiological, functional, and neurological characteristics throughout the drug development process. The ability to visualize biological processes in the early stages of oncology drug development gives researchers a critical advantage when it comes to lead compound prioritization. This pre-clinical understanding guides researchers through the selection of promising targets, elimination of ineffective or off-target compounds, refining dosing strategies, and monitoring biodistribution. Non-invasive preclinical oncology imaging provides biological feedback across a number of areas, discussed below.
- Pharmacokinetics – Preclinical oncology imaging enables direct, in vivo tracking of a drug’s absorption, distribution, metabolism, and excretion, namely pharmacokinetics. Pharmacokinetics has an extremely important role in early drug development and contributes to trial success and treatment discovery. Essentially, the main goal of preclinical trials is to identify promising compounds and identify a safe and effective dosing regimen, which would be almost impossible without an understanding of pharmacokinetics.
- Biodistribution – Biodistribution is a method of monitoring and analyzing the movement and distribution of specific compounds within a subject, including compound targeting, accumulation, and clearance. Without an understanding of biodistribution, it is extremely difficult to assess and predict the efficacy and potential toxicity profiles of a compound. Biodistribution studies provide valuable information on therapeutic localization, systemic exposure, and the assessment of off-target effects. For this reason, biodistribution studies are often required or, at the very least, strongly recommended by regulatory agencies, particularly when considering complex or targeted oncology therapies.
- Target engagement – Target engagement measures if and to what extent a compound binds to its intended target. Issues with target engagement cause a significant amount of drug development failure. According to a study published in Science Translational Medicine, off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials, a well-documented cause of clinical trial failure in oncology. Validating target engagement in preclinical studies is an essential component of clinical trial success.
Preclinical oncology imaging is an important intermediary step in the drug development process, acting as a refinement tool before moving into costly efficacy studies. Preclinical oncology imaging provides key biomarkers, understanding of pharmacokinetics and biodistribution, and informs target engagement and decision-making. Approximately 61% of oncology preclinical drug studies now utilize molecular imaging to track tumor progression and drug response, with the preclinical imaging market expected to reach a staggering 4 billion by 2033. Preclinical oncology imaging is no longer a complementary tool, but an essential component of oncology drug development.
Preclinical imaging modalities
A number of different imaging modalities make up preclinical oncology imaging, each with unique uses and advantages. Imaging modalities include:
- PET – Preclinical PET (Positron emission tomography) is a non-invasive technique that uses radioactive tracers to detect specific molecular pathways and cellular functions. Preclinical PET imaging provides valuable insights into drug efficacy, receptor binding, molecular targeting, biodistribution, and metabolism.
- MRI – Preclinical MRI (Magnetic resonance imaging) enables the visualization of anatomical structures and physiological functions via a strong magnetic field and radio waves. MRI provides high-resolution images of soft tissues, illuminating the subject, which enables the researcher to study the organs and structures within the body. Preclinical MRI imaging provides valuable insights into tumor volume, perfusion, and tissue characterization.
- Optical Imaging – Optical imaging is typically divided into fluorescence (FLI) and bioluminescence (BLI), meaning researchers can visualize biological processes at a molecular level with either external fluorescent dye or internal bioluminescent reporter genes and an external light source. Optical imaging is extremely sensitive, able to detect molecular events in the 10–15 M range, and ideal for high-throughput screening, gene expression studies, and tumor progression tracking.
- CT – Preclinical CT (Computed tomography) uses X-ray techniques to produce detailed images of the body. These high-resolution images provide essential information about tumor growth, metastasis, and treatment response, allowing researchers to analyze the effect of potential therapeutic compounds.
Smarter Go/No-Go decision making
Using non-invasive pre-clinical imaging techniques often means animals can be repeatedly scanned over time without the need for surgery or harm, reducing inter-subject variability and the number of animals needed. Combining different imaging modalities allows for a comprehensive understanding of tumor behavior, providing an early indication of therapeutic effects to enable rapid and viable go/no-go decisions during drug screening. Preclinical oncology imaging is an essential tool for oncology drug development, acting as a translational bridge to guide and optimize clinical biomarker imaging and personalize oncology therapies, enhancing confidence in candidate viability before IND (Investigational New Drug) submission. Failed oncology trials cost around $50-60 billion each year. Preclinical oncology imaging has the potential to significantly reduce this failure rate and ultimately contribute to the development of life-saving therapies for those who need them.
Accelerating innovation through strategic imaging partnership
Working with a preclinical oncology imaging partner who understands the importance and can facilitate the logistics of effective imaging modalities could be instrumental in the success of a scientist’s research.
One possible imaging partner is Perceptive Discovery, who utilize a comprehensive array of non-invasive preclinical imaging techniques to support drug development across various therapeutic areas, including oncology. Perceptive Discovery helps to bridge the gap between preclinical insight and clinical trial success, ensuring rigorous scientific analysis and high-quality data to support innovation.
Harness early insight, improved decision-making, and better translation. Learn more about Perceptive Discovery Services today, or contact a Discovery solutions specialist.
Header Image Source: Wikimedia Commons (image of heptamethine dye on a tumor in a mouse)
Edited by Jessica Desamero
Resources
WHO. Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer
Cell. Cancer biomarkers. https://pmc.ncbi.nlm.nih.gov/articles/PMC7616034/
StatPearls. Pharmacokinetics. https://www.ncbi.nlm.nih.gov/books/NBK557744/
Taylor & Francis. Biodistribution. https://taylorandfrancis.com/knowledge/Medicine_and_healthcare/Medical_genetics/Biodistribution/
Science Translational Medicine. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. https://pmc.ncbi.nlm.nih.gov/articles/PMC7717492/
Global Growth Insights. Preclinical Imaging Market. https://www.globalgrowthinsights.com/market-reports/preclinical-imaging-market-114953
JAMA. Costs and Causes of Oncology Drug Attrition With the Example of Insulin-Like Growth Factor-1 Receptor Inhibitors. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2807710#xd_co_f=NmJjYTRkNTYtMTk3OC00NDdjLWJkMTUtNWUyN2E2MGVkNTkx~

Leave a comment