Reading time: 5 minutes
Preeti Prangya Panda
In 1895, Wilhelm Roentgen discovered X-rays that paved the way for seeing into the human body. This invention revolutionized medical science. Today, the discovery has transformed into a powerful decoding tool that translates the hidden images in clinical scans. This emerging field is called radiomics, which is cracking the secret code for tumors and helping doctors predict and diagnose cancer early.
In simple words, radiomics can turn any scan into a treasure map guiding personalized care. That’s the oath of radiomics—a story of innovation that’s just beginning to advance.
According to the National Cancer Institute, 20 million new cases were detected in 2022. This value could rise to 30 million in 2040. However, studies have also shown that early detection of certain cancers can improve the survival rate by up to 90%. In this context, radiomics has great potential in the era of personalized cancer care.
What is Radiomics?
Radiomics is the science of extracting high-throughput quantitative data from medical images by using techniques like computed tomography (CT) and magnetic resonance imaging (MRI). These techniques use artificial intelligence (AI), machine learning (ML) and deep learning (DL) to scan the data and provide visually appealing images. While traditional imaging can see physically visible images, radiomics produces patterns and variations that are invisible to radiologists.
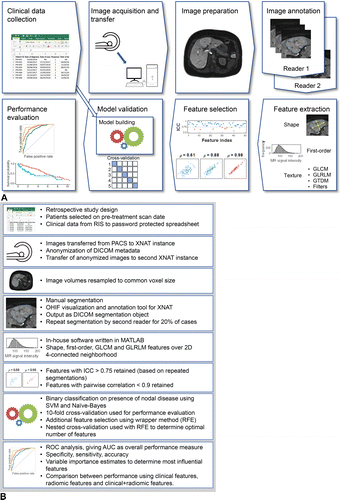
Source: https://pubs.rsna.org/cms/10.1148/rg.2021210037/asset/images/medium/rg.2021210037fig4.gif
The radiomic workflow aids in:
- Image acquisition or preprocessing: Radiomic analyses can be applied to various imaging modalities, including CT, PET, MRI, and US, with CT and PET offering inherently quantitative signal intensities and CT being less prone to motion artifacts.
- Tumor segmentation: Segmentation involves drawing regions of interest (ROIs) on the tumor, its subregions, or peritumoral zones based on the research hypothesis, with habitat imaging analyzing intratumoral heterogeneity and peritumoral zones providing insights into tumor invasion or immune response.
- Feature extraction: Feature extraction computes radiomic features from each ROI before model building and validation.
- Model development and validation: Statistical models use radiomic and clinical features to predict study endpoints like tumor type or survival time. Their performance and generalizability are assessed by validating them on new test data.
It also enables collaboration across disciplines such as radiology, imaging science and data science.
Statistical models classify patients into risk groups based on clinical outcomes like survival and time-to-event analysis. In contrast, radiomic data capture information about tumor biology, including temporal (with time) and spatial (with location) heterogeneity. This factor influences tumor behavior and therapy resistance. The detailing captured in radiomics makes it a potential “virtual biopsy,” offering noninvasive imaging that analyzes the entire tumor and enables easier monitoring over time, compared to traditional biopsies1.
Importance of Radiomics in Oncology
Radiomics assists in differentiating benign masses from malignant tumors by enhancing the detection rates. For example, in a study for breast cancer, researchers found that advanced techniques like contrast-enhanced mammography (CEM) and multiparametric MRI (mpMRI) are used along with the radiomic-clinical models to reduce biopsies and increase classification accuracy. After making the distinction between malignant and benign tumors, radiomics can grade tumors to differentiate low-grade malignancies from high-grade malignancies2.
It also identifies molecular subtypes and genetic mutations like ISH, KRAS, and 1p/19q codeletion using various imaging features like MRI and PET. By combining AI models like convolutional neural networks and random forest classifiers with radiomics, one can achieve high accuracy in enhancing imaging features of tumor biology and precision medicines.
Radiomic features predict responses to therapies like chemotherapy and immunotherapy. Some integrative models that combine the imaging, genomic and clinical data can improve prognostic accuracy and thus enable personalized treatment strategies. These features also aid in uncovering intratumoral heterogeneity (ITH) and tumor microenvironment (TME) like necrosis, edema and immune activity, which is an important tool for prognosis and therapy selection.
AI-powered radiomics improves diagnostic accuracy through automated tumor segmentation, classification, and grading. Techniques like deep learning and sparse representation-based radiomics outperform conventional methods, enabling precise identification of tumor types and molecular markers.
Radiomics links imaging features to tumor biology, predicting metabolic alterations, cell proliferation, and apoptosis. It further helps in treatment selection, improves therapeutic outcomes, and enables non-invasive evaluation of tumor biology. The prepared AI models predict metastatic potential, including lymph nodes and distant metastases, with high precision. Imaging features combined with machine learning reduce reliance on invasive procedures and support effective clinical management2.
Challenges Associated with Radiomics
Radiomics faces several challenges in its integration into clinical decision support systems, including the lack of standardization across imaging modalities and processing techniques, leading to variability, bias, and challenges in reproducibility. Overfitting in predictive models and the need for multicentric validation further complicate the development of robust, generalizable tools. Data privacy and security remain critical concerns, as accessing large datasets containing sensitive patient information requires balancing confidentiality with effective utilization. The interpretability of machine learning models, especially the “black box” nature of deep learning algorithms, hinders clinical adoption by making decision rationales difficult to understand3.
Future Directions and Emerging Trends
Future directions include an advanced understanding of AI algorithms to enhance accuracy and robustness, enabling personalized and adaptive treatment plans, a real-time adaptation of radiotherapy regimens, integrating multi-modal imaging, and adopting a multi-omics approach to provide a more comprehensive understanding of tumor biology.
As we stand on the brink of this exciting frontier, it’s crucial to invest in radiomics research and technology. By doing so, we not only improve lives today but also pave the way for a brighter, cancer-free tomorrow.
Header Image Source: https://www.heute.at/i/neue-ki-technologie-verbessert-diagnose-bei-krebs-120091841/doc-1ikf1rerh4
In-Article Image Source: https://pubs.rsna.org/cms/10.1148/rg.2021210037/asset/images/medium/rg.2021210037fig4.gif
Edited by Jessica Desamero
References
- Shur JD, Doran SJ, Kumar S, Ap Dafydd D, Downey K, O’Connor JPB, Papanikolaou N, Messiou C, Koh DM, Orton MR. Radiomics in Oncology: A Practical Guide. Radiographics. 2021 Oct;41(6):1717-1732. doi: 10.1148/rg.2021210037. PMID: 34597235; PMCID: PMC8501897.
- Qi YJ, Su GH, You C, Zhang X, Xiao Y, Jiang YZ, Shao ZM. Radiomics in breast cancer: Current advances and future directions. Cell Rep Med. 2024 Sep 17;5(9):101719. doi: 10.1016/j.xcrm.2024.101719. PMID: 39293402; PMCID: PMC11528234.
- Russo L, Charles-Davies D, Bottazzi S, Sala E, Boldrini L. Radiomics for clinical decision support in radiation oncology. Clin Oncol (R Coll Radiol). 2024 Aug;36(8):e269-e281. doi: 10.1016/j.clon.2024.03.003. Epub 2024 Mar 15. PMID: 38548581.


Leave a comment