Reading Time: 4 minutes
Chris Wang
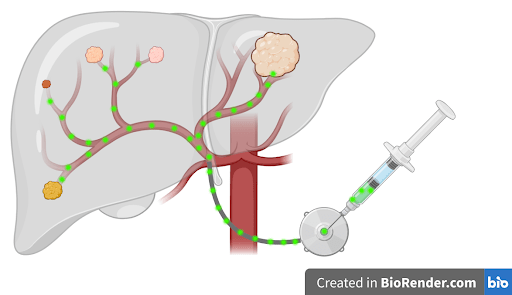
The liver is a uniquely important organ in your body. It filters all of your blood, produces bile to digest foods/carry away waste, and can even regenerate even after 90% has been removed. Unfortunately, the liver is also an organ where many cancers metastasize to. Colorectal cancer in particular often spreads through the blood into the liver via the portal vein. While healthy liver cells derive their oxygen from the portal vein, colorectal liver metastases (CRLM) require the higher levels of oxygen from the hepatic artery. This difference in oxygen required by normal cells versus cancer cells allows for localized treatments such as hepatic arterial infusion (HAI) chemotherapy.
Conventional chemotherapy is given intravenously, but this route causes off-target systemic therapy such as bone marrow suppression and gastrointestinal toxicity. Oncologists have long been interested in administering chemotherapy intra-arterially to deliver higher doses of chemotherapy selectively into the tumor. Researchers hypothesized that by administering chemotherapy through the hepatic arterial, systemic toxicity could be minimized and regional control of liver metastases could be maximized. In the 1980s, floxuridine (5-FUDR) became the ideal chemotherapy agent to administer through HAI due to its short half-life and high rate of conversion to inactive metabolites after passing through the liver. This meant that intra-hepatic floxuridine could kill CRLM but be quickly metabolized within the liver into inactive byproducts.
A limitation of administering drugs into an artery rather than a vein is that the pressure within arteries is higher than that in veins. Arterial injections require the use of an external pump. However, initial HAI pumps designed in the 1960s to 1970s were rudimentary and resulted in severe morbidity and mortality. Complications of HAI included air embolism, drug migration outside of the hepatic artery resulting in gastritis and bleeding, and hepatic injury including irreversible biliary sclerosis. Additionally, external HAI devices reduced patient mobility and required significant maintenance. To overcome these limitations, researchers in the 1980s to 1990s developed implantable HAI devices with self-contained power sources and silicone catheters that allowed for fewer lifestyle restrictions and decreased thrombosis rates. Additionally, surgeons refined their techniques to prevent drug migration into the gastroduodenal artery and improve post-op recovery times with laparoscopic approaches. Finally, medical oncologists developed standardized guidelines on dosing and co-administration of 5-FUDR with corticosteroids to help mitigate the risk of hepatotoxicity.
Since the late 1980s, multiple HAI pump clinical trials have shown benefit in improving disease control in the liver, delaying or preventing recurrence after liver resection, and increasing overall survival for metastatic colorectal cancer with liver-only metastases. Summaries of selected clinical trials are listed in Table 1. While initial trials showed promise, adoption of HAI chemotherapy has not been widespread. Limitations of the initial studies included use of outdated regimens, single-center trial design at high risk for bias, risk of irreversible biliary toxicities, and even inconsistent supply of HAI pump devices. Additionally, not all patients are eligible for HAI chemotherapy. Placement of the HAI pump requires a complex surgery involving arterial manipulation, and costs of acquiring and maintaining an HAI device are estimated to exceed tens of thousands of dollars per year. Only a few cancer centers across the United States are trained and able to implant an HAI pump and offer HAI therapy.
| Year | Study Author | Patient Population | Intervention | Control | Outcomes |
| 1987 | Kemeny N, et al. | N=162 Unresectable CLRM | 5-FUDR via HAI | 5-FUDR via IV | Higher response rate with HAI vs IV administration (50% vs 20%) |
| 1999 | Kemeny N, et al. | N=156 CLRM after liver resection | 5-FUDR via HAI+5-FU IV | 5-FU IV alone | Higher median overall survival with combination HAI+IV vs IV alone (72.2 vs 59.3 months) |
| 2006 | Kemeny N, et al. | N=135 Unresectable CRLM | 5-FUDR via HAI | 5-FU IV alone | Higher median overall survival with HAI vs IV administration (24.4 vs 20.0 months) Higher response rate with HAI vs IV administration (47% vs 24%) |
| 2015 | D’Angelica MI, et al. | N=49 Unresectable CRLM | All patients received the same treatment5-FUDR via HAI + systemic chemotherapy (5-FU + other agents) | 47% of patients were able to convert to resectable disease Response rate was 76% Median overall survival was 38 mo | |
Table 1. Summary of Selected Clinical Trials on HAI Chemotherapy for CRLM
Therefore, more multicenter, randomized controlled trials studying HAI therapy are needed. To answer whether the benefits outweigh the downsides to HAI chemotherapy, three large trials are ongoing. These include the PUMP, PUMP-1, and EXCALIBUR-1/2 trials in North America, the Netherlands, and Norway, respectively. Hopefully, with positive outcomes from these trials the role of HAI therapy can become more well defined. Until then, it remains a localized therapy for CRLM that only a limited number of specialized cancer centers may offer.
Header Image Source: Created by author in Biorender.com
Edited by Karli Norville
Abbreviations
CRLM: colorectal liver metastases
HAI: hepatic arterial infusion
5-FUDR: floxuridine
5-FU: fluorouracil
References
- Anteby R, et al. Getting Chemotherapy Directly to the Liver: The Historical Evolution of Hepatic Artery Chemotherapy. J Am Coll Surg. 2021 Mar;232(3):332-338.
- Kemeny N, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987 Oct;107(4):459-65.
- Kemeny N, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999 Dec 30;341(27):2039-48.
- Kemeny NE, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006 Mar 20;24(9):1395-403.
- DʼAngelica MI, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015 Feb;261(2):353-60.

Leave a comment