Reading time: 5 minutes
Kelby Kane
The role of tumor vasculature in tumor progression
It is becoming increasingly recognized that two hallmarks of cancer – the formation of new blood vessels (angiogenesis) and the suppression of your body’s ability to fight infections and other forms of damage (immunosuppression) – coordinate with each other to promote tumor growth and metastasis. Blood vessels within the tumor control the ability of immune cells such as cytotoxic T cells to move into the solid tumor and kill tumor cells. This happens in three ways; first, tumors stimulate uncontrolled growth of new blood vessels (angiogenesis) through the production of a molecule called VEGF. Second, these new vessels often lack receptors that help immune cells move out of the bloodstream and into the tumor. Finally, areas of the tumor grow so rapidly that blood vessel growth cannot keep up, and these areas eventually lack oxygen. This lack of oxygen triggers immunosuppressive signals within the tumors that keep the body from fighting the cancer cells.
In light of the interplay between the chaotic tumor blood vessels limiting effective T cell killing of the tumor, the idea of targeting both of these through a combination of immune checkpoint blockade (ICB) and inhibitors of angiogenesis could enhance and sustain the ability of T cells to infiltrate and activate and successfully eradicate the tumor. Interestingly, the combination of ICB plus a VEGF inhibitor can induce structural changes in the tumor vessels from flat to cubical, which is characteristic of cells known as high endothelial venules (HEVs).
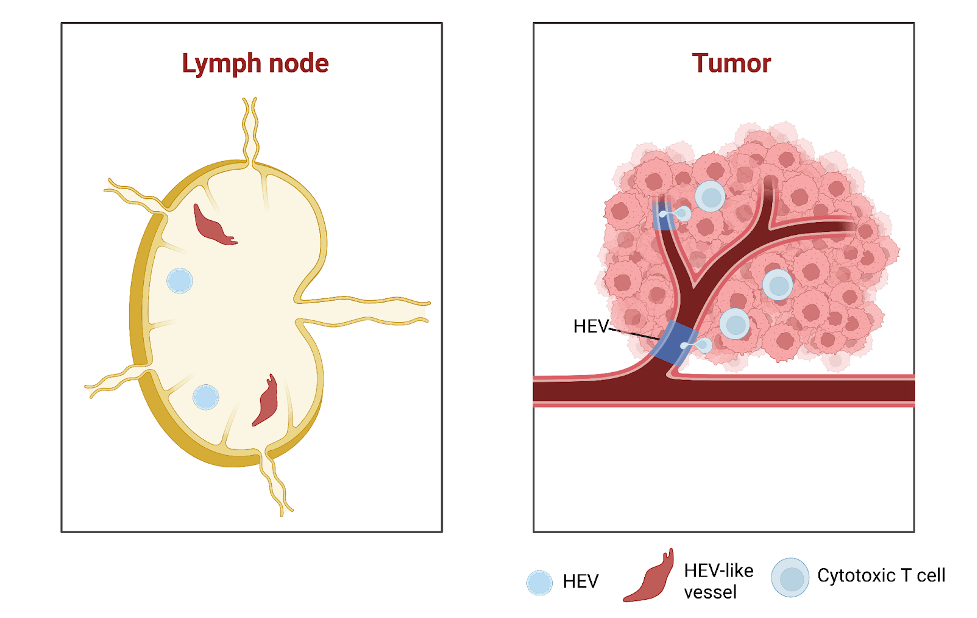
High endothelial venules in normal conditions
What exactly are HEVs? These specialized cells are normally found in organs such as the lymph nodes and tonsils and they play an important role in the production of T cells. The morphology of HEVs are distinct from endothelial cells, as HEVs are cuboidal and surrounded by a thick basement membrane and a perivascular sheath made of mural and reticular cells. They play an essential role at these sites to recirculate lymphocytes and activate the immune system against foreign invaders. In cases of inflammatory conditions, HEVs can develop at sites known as tertiary lymphoid organs. These structures are aggregates of lymphocytes that form outside of secondary lymphoid organs under chronic inflammation.
Figure 1. Showing the morphological differences between normal endothelial cells that line blood vessels and high endothelial venules. Image created with BioRender.
HEVs in cancer: a double-edged sword
HEVs can sometimes form in solid tumors, and their presence and abundance in tumors has been associated with increased disease-free survival and decreased metastasis in multiple cancers. The therapeutic induction of HEVs by the combination of angiogenic inhibitors and ICB could enhance anti-tumor immunity and efficacy. Tumor-HEV associated tertiary lymphoid organs are most commonly located outside the tumor and are rarely in the center. A recent study used single-cell sequencing to map the fate of HEvs and discovered that tumor endothelial cells located at the smallest blood vessels known as postcapillary venules are the cell type that transform into HEVs upon therapeutic induction using a combination approach of anti-angiogenic therapy and ICB.
However, while HEVs can promote T cell infiltration, these structures in lymph nodes may concomitantly also serve as areas where cancer cells can disseminate to from the primary tumor. The first lymph nodes cancer cells colonize are called sentinel lymph nodes, and in a cancer diagnosis are examined to determine whether cancer has metastasized. Interestingly, HEVs at these locations seemed to undergo remodeling that resulted in them having dilated vessel lumens and a flatter morphology, reminiscent of endothelial cells. These remodeled sentinel lymph nodes were found to be associated with decreased overall survival in patients with tongue squamous cell carcinoma.
Conclusion
In summary, recent preclinical studies examining the effects of combination therapies targeting tumor angiogenesis and immunosuppression has shown that the induction of tumor-associated HEVs could further improve the eradication of tumors. Despite the presence of these specialized structures potentially posing favorable outcomes and their therapeutic formation facilitating anti-tumor immunity, more research needs to be done on their role in cancer progression and how they may vary across different cancer types and immune states in preclinical models and in human patients. It seems these structures can have dual effects on enhancing anti-tumor immunity or facilitating cancer cell spread through sentinel lymph nodes. Nonetheless, future research on better understanding and targeting HEVs could be an attractive feature to target due to their ability to enhance lymphocyte recruitment and potentially pose more favorable outcomes.
References
- Allen, E., Jabouille, A., Rivera, L. B., Lodewijckx, I., Missiaen, R., Steri, V., Feyen, K., Tawney, J., Hanahan, D., Michael, I. P., & Bergers, G. (2017). Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Science Translational Medicine, 9(385). https://doi.org/10.1126/scitranslmed.aak9679
- Hua, Y., Vella, G., Rambow, F., Allen, E., Antoranz Martinez, A., Duhamel, M., Takeda, A., Jalkanen, S., Junius, S., Smeets, A., Nittner, D., Dimmeler, S., Hehlgans, T., Liston, A., Bosisio, F. M., Floris, G., Laoui, D., Hollmén, M., Lambrechts, D., … Bergers, G. (2022). Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches through a feed-forward loop. Cancer Cell, 40(12). https://doi.org/10.1016/j.ccell.2022.11.002
- Hussain, B., Kasinath, V., Ashton-Rickardt, G. P., Clancy, T., Uchimura, K., Tsokos, G., & Abdi, R. (2022). High endothelial venules as potential gateways for therapeutics. In Trends in Immunology (Vol. 43, Issue 9). https://doi.org/10.1016/j.it.2022.07.002
- Vella, G., Guelfi, S., & Bergers, G. (2021). High Endothelial Venules: A Vascular Perspective on Tertiary Lymphoid Structures in Cancer. In Frontiers in Immunology (Vol. 12). https://doi.org/10.3389/fimmu.2021.736670
- Vella, G., Hua, Y., & Bergers, G. (2023). High endothelial venules in cancer: Regulation, function, and therapeutic implication. In Cancer Cell (Vol. 41, Issue 3). https://doi.org/10.1016/j.ccell.2023.02.002

Leave a comment