Reading time: 7 minutes
Gracie Jennah Mead
Introduction
Macrophages are an important part of the innate immune system. Within the innate immune system, which comprises more indiscriminate strategies that protect us from pathogens in general, macrophages engulf and break down pathogens. Macrophages are a diverse cell as they can regulate tissue development and tissue repair after injury and engulf pathogens [1]. More recently macrophages have been a point of interest in cancer biology. Tumour associated macrophages (TAMs) have been found to be one of the most abundant infiltrating cells into the tumour [1] and are important for tumour immunity, progression and metastasis. Additionally, in patients with a large presence of TAMs has been linked with poor prognosis in patients. This apparent influence on patient outcomes makes macrophages a very interesting target for future therapeutics.
What are M1 and M2 macrophages?
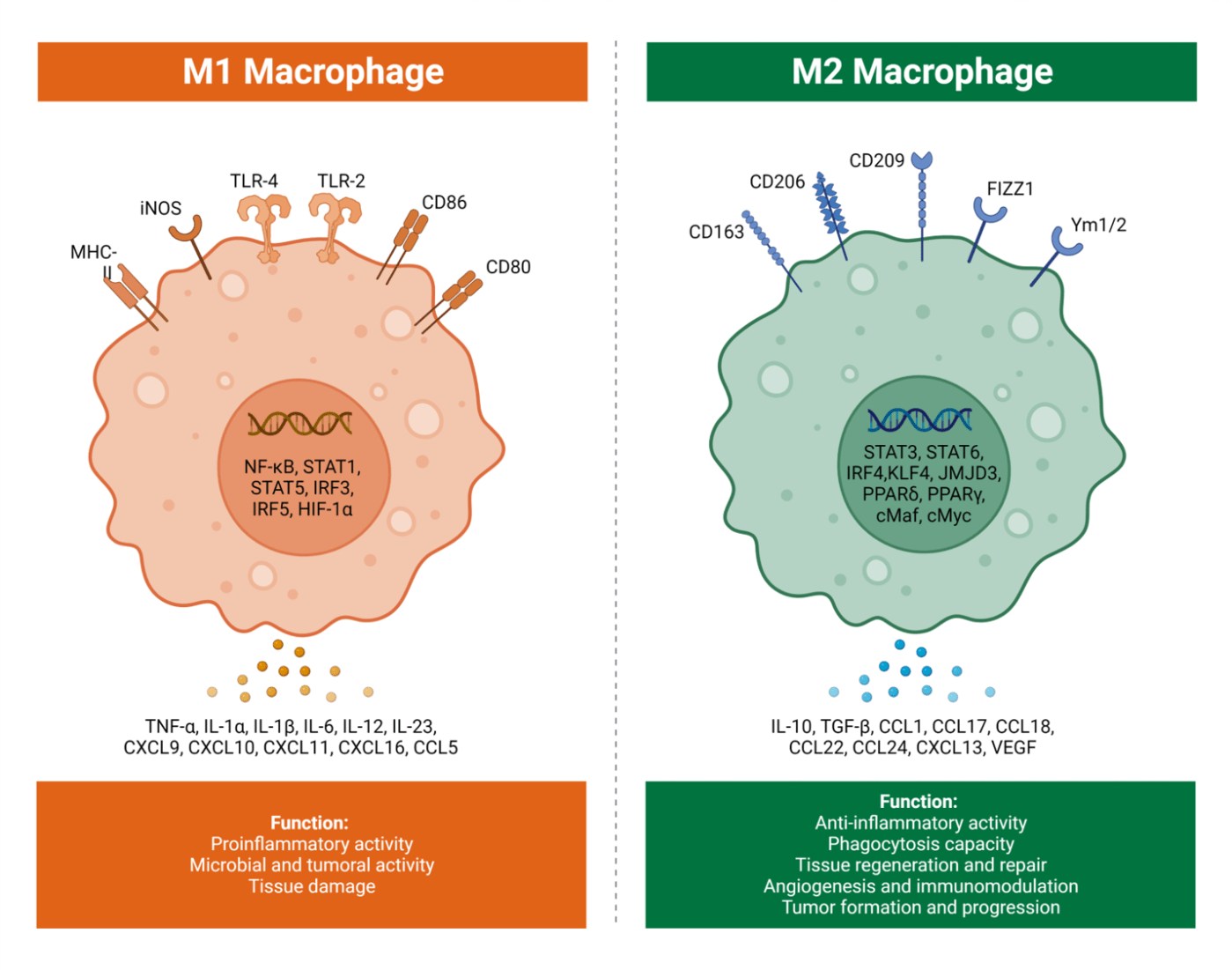
M1 and M2 macrophages were first discovered in the early 2000s, where researchers discovered that there were different subtypes of macrophages and that they exhibited different functions. The discovery was based on previous work that distinguished T cell immune responses into T helper 1 (Th1) and T helper 2 (Th1), with Th1 being more pro-inflammatory and Th2 being more anti-inflammatory. This initiated the discovery that macrophages have different subsets, called M1 and M2. Much like T helper 1 and T helper 2 responses, M1 is more pro-inflammatory and M2 is more anti-inflammatory [1]. M1 macrophages can be differentiated from naive macrophages (M0) when in the presence of interferon gamma (IFNg) or bacterial lipopolysaccharide (LPS). These two stimuli lead to M1 macrophages being differentiated which then express inflammatory factors such as interleukin 1 beta (IL1b), IL6 and tumour necrosis factor alpha (TNFa). M1 macrophages exhibit pro-inflammatory effects and anti-tumour responses [2]. Conversely, M2 macrophages differentiate from M0 when in the presence of IL4 and IL13. M2 macrophages are anti-inflammatory as they produce anti-inflammatory cytokines IL10 and transforming growth factor beta (TGFb), and are involved in tumour initiation, proliferation, metastasis and immune evasion [2].
M2 macrophages importance in cancer
Macrophages which infiltrate the tumour microenvironment (TME) are known as tumour associated macrophages (TAMs). TAMs have predominantly a M2 phenotype and function [2]. M2 macrophages are important with regards to tumour progression as they are anti-inflammatory and have pro-tumorigenic functions which makes them a potential target for cancer therapeutics. The pro-tumorigenic functions are divided into three groups: immunosuppression where the immune response is limited, angiogenesis where new blood vessels are formed and metastasis where tumour cells move from their original location and grow in a new location. M2 macrophages in the TME express CD206, is a receptor typically expressed by macrophages and dendritic cells. Under normal circumstances CD206 is important in phagocytosis, but when expressed by TAMs it is seen as a biomarker for poor patient outcomes. Additionally, M2 macrophages in the TME express CD163, which is another type of receptor that correlates with poor outcomes for patients. M2 macrophages also secrete vascular endothelial growth factor (VEGF) which promotes tumour growth through tumour cell expansion, migration for metastasis, longer survival and increased vascular permeability which enables the tumour cells to move to a new location in a process known as metastasising [3]. VEGF also supports the formation of new blood vessels in and around the tumour (a process known as angiogenesis) which helps deliver nutrients to the tumour to support further growth. All of these new blood vessels provide more pathways for the tumour to relocate and grow [3]. Finally, M2 macrophages also secrete IL10, which stimulates tumour growth through activating signalling pathways that inhibit apoptosis in tumour cells, preventing them from being killed. IL10 also can cause immune cell escape which is when dendritic cells are inhibited to downregulate the immune response leading to immunosuppression as tumour antigens are downregulated reducing the chances of tumour cells being detected and killed by immune cells [4].
M1 macrophages importance in cancer
There is a smaller proportion of M1 macrophages in the TME compared to M2 macrophages. However, the M1 macrophages we know are more pro-inflammatory, and this is still the case in the TME [2]. The M1 macrophages present in the TME express CD80 and CD86, which are both types of receptors that initiate signals for T cell activation. Expression of CD80 on M1 macrophages in the TME allows natural killer (NK) cells to recognize and break down the membrane of tumour cells aiding their killing. CD80 also plays a role in costimulation of T cells, which is when CD80 binds to CD28 expressed on T cells to provide a secondary signal to help them produce IL2, a cytokine which helps T cells to proliferate aiding tumour cell killing. Additionally, CD86 also provides costimulatory signals to T cells through binding CD28 on T cells for their survival and activation which can increase tumour cell killing and ultimately tumour immunity [5][6].
Figure 1: Showing the differences between M1 and M2 macrophages, with receptors, genes, functions and cytokines. Taken from [7]
How TAMs are being targeted for therapeutics in cancer?
Tumour associated macrophages have posed as a very good target for cancer therapeutics as they contribute to the immunosuppressive environment of the TME but also show the ability to be moulded and altered with different functions and phenotypes as can be seen between M1 and M2 macrophages. Because of this ability to be altered therapeutics have focused on repolarizing TAMs to a more M1, pro-inflammatory phenotype. The strategies for targeting TAMs have included blocking monocyte recruitment. Monocytes are the starting cell before they are matured and are fully functioning macrophages. There is a component called CCR2, which is a receptor expressed on monocytes and these CCR2 expressing monocytes are recruited by the tumour and then the monocytes mature into TAMs The idea is that blocking CCR2 with an antibody may prevent these monocytes from being recruited and reduce the amount of TAMs in the TME and reduce the anti-inflammation [8]. Other therapeutics such as blocking the epidermal growth factor receptor (EGFR) which is responsible for the upregulation of VEGF, which we know supports tumour cell growth, metastasis and angiogenesis. Blocking EGFR on TAMs has been shown in mice to reduce the growth of ovarian cancer in mice[8].
Conclusion
Tumour associated macrophages are a very important part of tumour immunity. Their ability to alter phenotype and function between M1 pro-inflammatory and M2 anti-inflammatory make them an interesting and exciting target for cancer therapeutics. Targeting TAMs is now a very prominent topic of research for finding new ways to treat cancer, there have already been many advancements such as anti-EGFR monoclonal blocking antibodies involved in blocking growth receptors and blocking monocyte recruitment to prevent macrophage maturation. Overall the area of macrophage research in tumour immunology is a very exciting endeavour.
Edited by Michael Marand
References
[1] Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023 Apr;23(4):238–57.
[2] Gao J, Liang Y, Wang L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol. 2022 Jun 30;13:888713.
[3] Verheul HMW, Pinedo HM. The Role of Vascular Endothelial Growth Factor (VEGF) in Tumor Angiogenesis and Early Clinical Development of VEGFReceptor Kinase Inhibitors. Clinical Breast Cancer. 2000 Sep;1:S80–4.
[4] Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023 Jun 8;14:1161067.
[5] Mir MA. Concept of Reverse Costimulation and Its Role in Diseases. In: Developing Costimulatory Molecules for Immunotherapy of Diseases [Internet]. Elsevier; 2015 [cited 2023 Nov 8]. p. 45–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128025857000029
[6] Mir MA. Introduction to Costimulation and Costimulatory Molecules. In: Developing Costimulatory Molecules for Immunotherapy of Diseases [Internet]. Elsevier; 2015 [cited 2023 Nov 8]. p. 1–43. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128025857000017
[7] Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017 Nov;117(11):1583–91.
[8] Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020 Dec 3;11:583084.