Reading time: 5 minutes
Chris Wang
Chemotherapy is a mainstay of cancer treatment, yet many times it is temporarily withheld or stopped due to significant toxicity. This is because chemotherapy drugs cannot differentiate between killing cancer cells and killing fast growing healthy cells, such as cells in your bone marrow. These cells divide quickly because they replace short-lived blood cells (see Figure 1). Red blood cells (erythrocytes) live for only about 100 days, platelets (clotting cells) live for a week, and white blood cells, including a subtype called neutrophils, typically live between a couple of hours to a few days. Because these cells die off so quickly, they are constantly being renewed from stem cells in your bone marrow. Because chemotherapy targets rapidly-dividing cells, a hallmark of cancer cells, this unfortunately means it also wipes out the fast growing stem cells in your bone marrow. When this happens, cancer patients can develop low red blood cells (anemia), low platelets (thrombocytopenia), and low white blood cells (neutropenia). Because red blood cells carry oxygen throughout the body, not having enough of them can cause patients to feel extremely tired or even be short of breath. For patients that have extremely low platelets, even a simple cut or bruise can cause a life-threatening bleed. When patients lose a specific type of their white blood cells called neutrophils, they become much more vulnerable to infections or have otherwise mild infections turn deadly.
To avoid this, many cancer patients treated with chemotherapy are treated with agents to lessen the effects of chemotherapy-induced bone marrow damage. To treat anemia, cancer patients can be given a hormone that helps stimulate RBCs to grow or be given a blood transfusion. However, using this hormone has been associated with a decrease in overall patient survival and blood transfusions carry risks of allergic reactions, so only extremely anemic patients are considered for treatment . When cancer patients present with low platelets, they can be given platelet transfusions or be given a stimulating hormone; however, this can introduce a risk of overshooting the goal platelet level and developing a clot. To treat low white blood cells, cancer patients can be administered a white-blood cell specific hormone that promotes neutrophils to grow. However, this hormone must be given at least 24 hrs after the completion of chemotherapy, which can result in additional visits to the oncology clinic, and is not always effective. Therefore, even with the availability of agents used to treat the loss of bone marrow stem cells, it would be better to prevent it from happening than to treat it once it has already occurred.
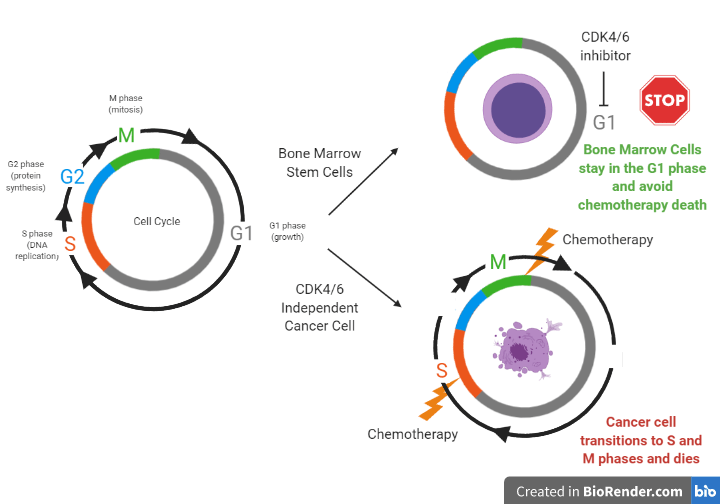
Therefore, the question becomes: how can we help chemotherapy target only the cancer cells instead of both the tumor and healthy bone marrow stem cells? Researchers at a spin off company from UNC-Chapel Hill, called G1 Therapeutics, have studied the use of a new drug, trilaciclib, to help do just that. Trilaciclib inhibits proteins that control cell division called CDK4 and CDK6 within bone marrow stem cells to temporarily stop them from growing. Since chemotherapy agents only target fast growing cells; if healthy cells are told to stop dividing before they are exposed to chemo, they can be spared. To see exactly how CDK4/6 inhibitors work to freeze bone marrow stem cells but not cancer cells from growing, we must first understand the phases of the cell cycle. Cell division can be divided into important phases of the cell cycle. Cells that are in the G1 (growth) phase are protected from harm from chemotherapy because chemotherapy only affects cells that are in the S (DNA replication) and M (cell division) phases. Different types of cells use different ways to transition between phases of the cell cycle to divide. For example, bone marrow stem cells use proteins called CDK4/6 to move from the G1 phase to the S phase while other cells, such as tumor cells from certain lung cancers, use a different mechanism (see Figure 2). When bone marrow stem cells are told to stop growing through exposure to a CDK4/6 inhibitor, they avoid moving from the G1 phase, while cancer cells continue to transition to the S and M phases to divide. When both the cancer and stem cells are exposed to chemotherapy, only the cancer cells die, since they are targeted as fast growing cells, while the bone marrow stem cells are spared.
While researchers at G1 Therapeutics found the mechanism from Figure 2 to be true in mouse models and human cell models outside the body, they had to prove this concept worked in real-life cancer patients to get FDA approval. And so they did just that; in a phase II (small safety/efficacy study) randomized clinical trial, researchers found administering trilaciclib intravenously right before treatment with toxic chemotherapy helped lower the bone marrow toxicity patients with a type of lung cancer (SCLC) experienced from chemotherapy compared to the placebo. Trilaciclib lowered the occurrence and duration of severe neutropenia and was associated with fewer rescue blood transfusions for anemia than placebo. Additionally, trilaciclib treatment did not prevent the chemotherapy from working; in fact progression free survival (the length of time before the tumor grows or cancer gets worse) was longer in some patients who were given this CDK4/6 inhibitor than placebo. Due to the success of this study, the company is currently filing a New Drug Application for trilaciclib in SCLC, with hopes of FDA approval in near future. The impact of preventing the limiting side effect of white blood cell suppression and the ability of this medication to help improve the quality of life for cancer patients by preventing chemotherapy toxicity provides enthusiasm for future treatment with CDK4/6 inhibitors.
Edited by Sara Musetti
Editors note: This article was corrected to change NSCLC to SCLC and update the progress of trilaciclib in clinical trials on June 11th. We apologize for the error.
Reference:
Weiss, J., Csoszi, T., Maglakelidze, M., Hoyer, R., Beck, J., Gomez, M. D., … Dragnev, K. (2019). Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Annals of Oncology, 30(10), 1613–1621. doi: 10.1093/annonc/mdz278
Additional info:
Link to previous article on CDK4/6 inhibitors. Past article talked about how CDK4/6 inhibitors may help sensitize non-responsive tumors to PDL-1 inhibitors.

Thanks alot for the nice article
LikeLike
Very good article
LikeLike
Thanks for sharing such an informative blog, the blog consists really a great stuff.
LikeLike
It was a very good article. Health to your hands
LikeLike
Thanks for all the research paper it was very informative
LikeLike
Very relevant content! 🙂 I have learned a lot from this article, thank you so much for sharing this!
LikeLike
Amazing a lot of beneficial info!. Many thanks.
LikeLike
Really like your blog , With thanks.
LikeLike